3 Ways CROs Can Expedite Clinical Trial Site Success
Streamline your operations and improve trial feasibility and outcomes.
Clinical Research Organizations (CROs) play an important role in the success of clinical trials. They provide crucial resources, processes, and strategies to ensure that trials are conducted with the highest standards of accuracy and data quality. CROs can empower trial success by leveraging technology-enabled processes, risk assessment strategies, and other approaches to identify high-performing sites, attract diverse populations to participate in clinical trials, and minimize administrative burden on Principal Investigators (PIs).
A recent article in Clinical Trials Arena stated that open data-sharing models and personalized medicine are quickly becoming industry standards for clinical trials, specifically those contracting with CROs.
In this blog post, we’ll explore how disparate data can help inform smarter trial site feasibility and recruitment decisions for better outcomes specifically for CROs but also for global trial success.
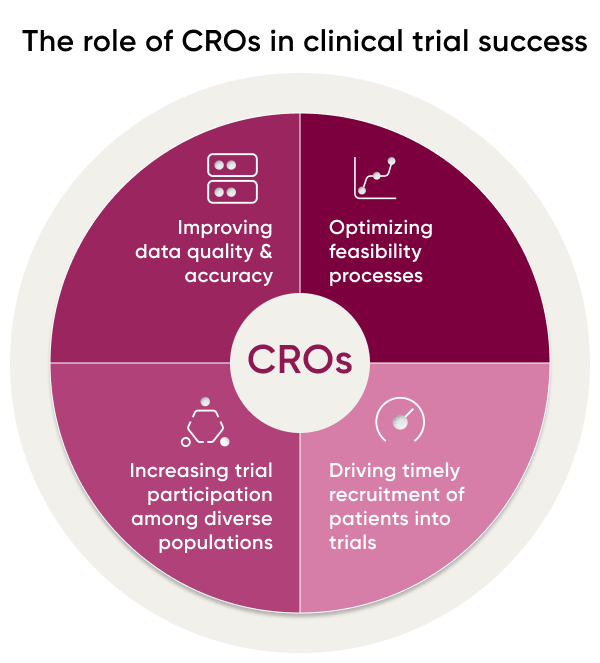
CRO Role in Trial Site Success & Feasibility
When it comes to the success of CROs, two key components are trial site feasibility and trial recruitment. Ensuring that trial sites have the infrastructure, capabilities, and personnel to support a successful study is critical, as is having adequate recruitment of diverse participants. With so much at stake in terms of time, money, and resources, it’s essential for CROs to employ data-based strategies to maximize their potential for success.
CROs can help improve trial site feasibility and success by increasing recruitment and boosting representation among study participants. They can offer services and resources, including study protocol development, clinical operations management, data management, safety monitoring, regulatory support, investigator selection and training, patient recruitment strategies, data analysis and reporting.
By leveraging their expertise and experience in conducting clinical trials, CROs can reduce the administrative burden on PIs so that they can focus on what matters most – providing high-quality care for patients.
This includes identifying high-performing sites and recruiting diverse populations for participation in a trial. To do this successfully requires technology-enabled processes as well as risk assessment strategies such as determining the likelihood of successful recruitment among different participant groups or evaluating potential sites based on past performance data.
When it comes to trial site feasibility, having access to data can help with identifying the best sites for a given study. Factors such as geographic location, patient population, and past performance of different sites need to be taken into account when assessing possible locations for a clinical trial. Having access to accurate and up-to-date information about potential sites can help ensure that CROs are making informed decisions about where studies should be conducted.
CROs & Trial Representation
Diversity is an essential element of any clinical trial’s success because it allows researchers to obtain results that accurately represent all members of a population being studied. However, historically underrepresented populations have often been excluded from participating in clinical trials due to logistical barriers or lack of awareness about study opportunities available within their communities.
In order to ensure that all populations are represented in research studies, CROs must create targeted recruitment strategies that effectively reach out to underserved populations and make study participation more equitable across different communities. This may involve developing collaboration networks with community partners or engaging with key stakeholders such as local healthcare providers or patient advocacy organizations that can help spread the word about available trials within their respective networks.
In addition to addressing diversity issues through customized outreach initiatives designed for various participant groups, CROs can also reduce administrative burden on PIs by providing automated systems for managing permissions requests from participating sites or organizing electronic submission of required documents from sponsors/investigators during the course of a study.
CROs and Trial Recruitment
Data is also an important part of the recruitment process. Knowing how potential participant demographics will fit into a study is essential in order to ensure that the results are as representative as possible. Access to demographic data can help CROs identify where there may be gaps in recruitment efforts and create strategies to close these gaps.
For example, if a particular trial has difficulty recruiting certain ethnic or racial groups, looking at detailed demographic data can help determine why this group may not be enrolling in sufficient numbers, so steps can be taken to address any barriers they may be experiencing. In addition, head-to-head comparisons between different sites or regions can give valuable insight into how different recruitment strategies may play out in each area.
Finally, patient representation must also be taken into account when considering recruitment strategies. Having access to patient data – such as social determinants of health – can provide valuable insights into which areas have greater healthcare disparities and which interventions may be most effective for those populations. This information can then be used by CROs in designing targeted recruitment strategies aimed at reaching underserved populations.
The use of these technologies can significantly streamline the process of coordinating activities between sponsors/investigators and sites while improving data accuracy by reducing errors associated with manual data entry or paperwork processing.
Finally, collecting and analyzing disparate data points is essential for successful trial site feasibility and recruitment processes for CROs. Not only does it provide valuable insights on the best sites for studies as well as how best to reach out to diverse communities; but it also helps ensure that all participants are treated fairly according to their needs and circumstances when enrolling in trials. By understanding how disparate data impacts CRO success, organizations will have the necessary knowledge and tools required for achieving maximum efficiency while adhering to ethical standards of care during the clinical research process.
CROs play an important role in facilitating successful clinical trials by providing crucial processes and resources necessary for achieving desired outcomes while reducing administrative burden on Principal Investigators. In order to ensure trial site feasibility and success, CROs must leverage technology-enabled processes as well as risk assessment strategies that identify high-performing sites while encouraging diverse participants from different backgrounds to join studies. In addition, CROs should develop tailored outreach plans designed specifically for reaching out to underserved communities so that all individuals are included in research studies regardless of socio-economic status or geographic location.
To learn more about how H1 can enable CROs for trial success, request a demo of our clinical trial intelligence platform.
