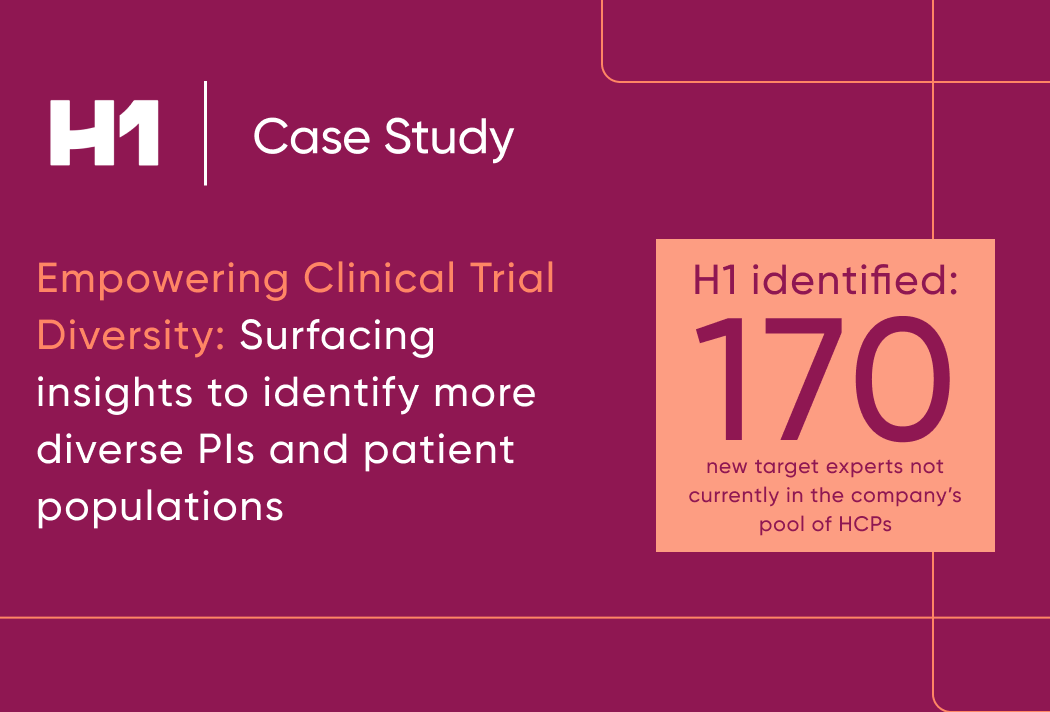
Empowering Clinical Trial Representation
Surfacing Insights to Identify More Inclusive PIs and Patient Populations
A new study presented at the American Heart Association’s Scientific Sessions 2022, and also being simultaneously published in JAMA: Journal of the American Medical Association, found significantly lower mortality for patients treated within guideline-recommended time goals for treatment of ST elevation myocardial infarction (STEMI), a type of heart attack caused by a complete blockage in a coronary artery.
However, the data identified concerning trends across the entire episode of care for patients experiencing STEMI, including increasing delays in treatment, greater numbers of untreated patients and increasing risk-adjusted in-hospital mortality and readmission rates.
The findings were shared at Scientific Sessions, a premier global exchange of the latest scientific advancements, research and evidence-based clinical practice updates in cardiovascular science.
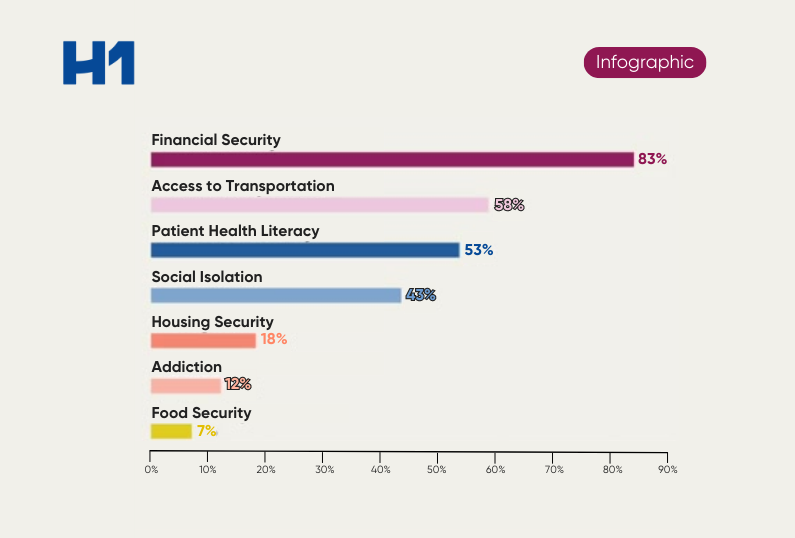
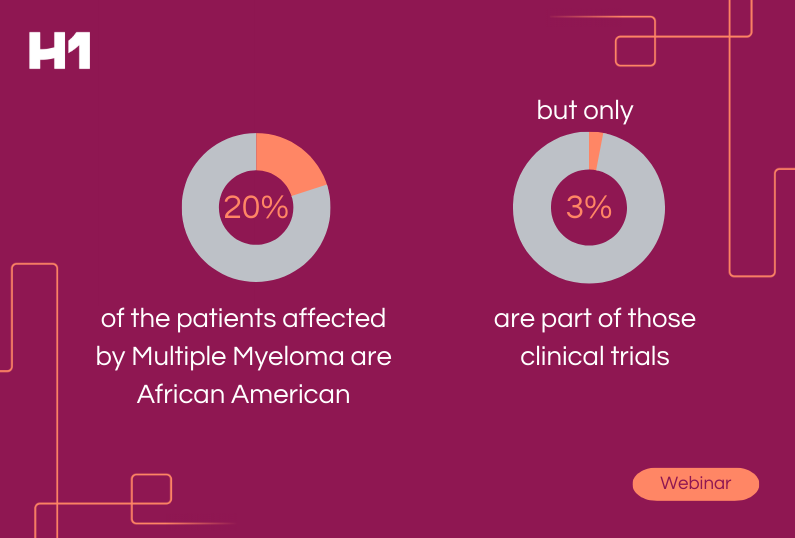
The study illustrates the dangers of gaps in care that can be traced back to the clinical research or lack of inclusion in cardiology. Very often, the populations that the drugs or studies aim to help and the physicians treating them are left out of critical research, causing an impact and gap in the intended purpose of research–improving quality of life for those affected most by the disease or condition.
Strategizing for Clinical Trial Representation
In this case study, like many top 10 and global pharma companies, an H1 client needed to diversify both its patient outreach and site investigator profiles for upcoming trials. The company had kicked off a new cross-enterprise focus on expanding their criteria for trial site principal investigators (PIs) and clinical trial site profiling.
To support ongoing therapy development, the company was looking to gain a holistic view of potential trialists to be able to best profile and identify principal investigators who fit the organization’s patient representation strategic business needs for systemic lupus erythematosus (SLE) in the U.S. H1 used a tech-first, data-driven, and cross-functional approach to provide a holistic understanding of trialists that best fit the ideal PI profile. Diversity metrics included: trial experience; relevant patient population D&I metrics; academic expertise; HCP historical performance, and HCP demographic information.
Highlight of Findings: The client utilized H1’s Clinical solutions and PI insight analysis to find:
- 170 net new HCPs who met the desired profile
- Net new patient populations and HCP demographics
- Identify and segment new HCPs who have a digital footprint
Learn more by downloading the case study below.