The Opportunities and Challenges Patient Representation with Expert Daniel Perez

The pursuit of better healthcare for all has many obstacles. Achieving a state where everyone has fair access to healthcare is a massive endeavor with many stakeholders. It requires addressing historical and modern injustices associated with economic and social factors to eliminate preventable health disparities.
With such a complex model, understanding all the pieces requires an expert perspective. In a recent interview, Daniel Perez, the Director and Global Head of Health Equity for Worldwide Clinical Trials shared insights from his personal and professional experiences. Worldwide Clinical Trials is a leading full-service global CRO (contract research organization) working with biotech and pharma to create customized solutions.
Perez spoke about the opportunities and challenges in clinical trial representation and the role of pharma companies and CROs to drive more inclusive research.
Perez Explored His Own Path
Perez has worked in the field for years but noted it wasn’t a deliberate career path. The beginnings of his career in healthcare came from a desire to heal. He considered the idea of healing to be more than just literal ailments of the body. He said, “It’s also the continuity of that health with your wellness of mental and emotional health.”
In the realm of improving healthcare for underserved communities, Perez shared that equity was the keyword to unpack. The trickiness of any equity involves the basic understanding that people don’t all get the same start in life. Perez noted that his own background had limitations, as he was raised by a single mom living paycheck to paycheck.
With this reality, he saw that the limitations of equity meant his mother couldn’t take the time to care for herself in terms of regular physician visits or preventative care screenings. He relayed that he had been able to help his mother rise from this state, and now she has the ability to focus on her care.
This was not the case for his mother’s sister, his aunt. She was unable to access regular healthcare visits, and by the time her pain became unbearable, it was too late. She had a large tumor and passed away from cancer soon thereafter.
This example highlighted that equity in health is hard to achieve. Perez’s aunt had economic, social, and accessibility obstacles. In addition to these, he also mentioned the health literacy gap. Neither his aunt nor his mother understood the importance of preventative care. They weren’t alone, as nearly nine out of 10 adults in the U.S. struggle with this.
Without health literacy, the gap in health equity grows. The experiences of his mother and aunt spurred bigger questions about how people become part of clinical trials. Perez said, “Why was someone like her not approached for a panel of clinical trials?” The answer to that question had many layers associated with it.
For Perez, health equity means zooming out and looking at the breadth of the dynamic and all the elements at play with simply receiving care. The starting point to this desired outcome of equity is having a wide aperture and understanding what the continuum looks like.
The Role of CROs
CROs play a vital role in patient representation, though significant challenges persist throughout clinical trial processes. These must be addressed system-wide to achieve the greatest impact. Perez called out the complexities of psychiatric clinical trials and the stigma still associated with mental health. Black Americans are another underserved group that disproportionately ends up in emergency rooms and are simply assigned an ICD-10 code regarding mental health conditions.
All of these things impact the prescreening that CROs conduct, and Perez shared examples of how to improve it. The first part is asking the sites what the prescreening process looks like. The second step is developing a prescreen modality that captures demographic data as well as why a person passed or failed the process.
This would seem like a simple question to ask a prospective patient for a trial if they have a diagnosis, but often, the patients who have no consistency in care don’t know the answer or can only say they were diagnosed with something in the ER.
FDA Planning with CROs and Sponsors
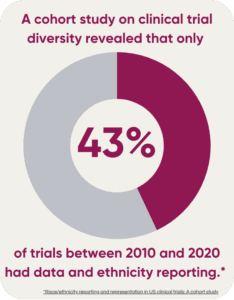
How diverse are clinical trials? A cohort study on clinical trial diversity revealed that only 43% of trials between 2010 and 2020 had data and ethnicity reporting. The researchers concluded that race and ethnicity reporting was insufficient, and enrollment of minority races was poor but improving.
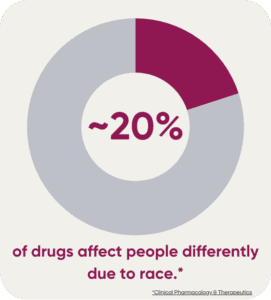
So why does all this matter? When a drug comes to market, it may not be safe or effective for all races. Around 20% of drugs affect people differently due to race. This could keep treatments out of reach for some of the most vulnerable populations.
The framework provided is a general framework requiring an action plan for diversity. Perez said he didn’t think the FDA’s intention was to invent a new wheel. Rather, the push was to connect the existing ingredients that make up the clinical trial.
FDA Diversity Planning must involve multiple stakeholders and not happen in silos. By coming together, Perez believes everyone can start thinking differently about clinical trials. As a functioning group, all the players can break things down into specific actions. The action plan must come before an enrollment plan.
By moving in this direction, Perez stated that diversity planning can become operationalized if you can successfully demystify the process and rely more on accurate data management.
Adapting FDA Guidance to the Specificity of the Trial
Next, Perez walked through what the application of the FDA rules looks like in a clinical trial. His approach included many questions to answer—What’s the country selection mix strategy? What is the sponsor’s commercialization goal?
Gathering the answers then helped him pivot and adjust the diversity plan. With more data and a focus on avoiding the things that make drugs fail, diversity can find a place in trials. Perez urged that sponsors should not use a lens of requirement for the application but rather build a comprehensive strategy that will encompass the entire continuum of the trial.
How Technology Plays a Key Role
Another component that has played a big role in improving global healthcare is technology. Perez described that the technology wasn’t of much use unless those applying it understood the problem first. “The best technology has to put the end user in the middle of it and is one that we don’t have to think about because it’s just there.” Ultimately, for technology to have the best impact, it must elevate and scale.
One of the most significant challenges in technology regarding diversity is cohesively bringing all the data and elements together. Ideally, protocols are reusable and adaptable, and an AI analytics engine can digitize them. The ability to plug in inclusion and exclusion criteria into a platform and have it deliver a cross-analysis of country, demographics, and population is essential.
Perez observed, “The industry right now is contract execution happy and friendly, and they move quickly. It is something that I have been a very balanced critic of.” Despite the tendency toward rapid execution, notable exceptions persist. “On the flip side, we see efforts of companies like H1 that are thoughtfully designed, not on the interface front first. And that’s the part and the distinction that I respect and admire,” he added.
On a broader perspective, Perez emphasized the importance of viewing solutions through a comprehensive lens. “Let’s back up and just think about this from a pathway perspective. Earlier, I spoke about what health equity looks like in that full continuum and pathway,” he suggested. He commended H1’s strategy, elaborating on their effective use of data:
“One of the things that has been admirable about H1 amongst several is that y’all have taken that spectrum and understood what data assets can actually lend value and unlock insight.”
By prioritizing data insights over superficial enhancements and leveraging valuable data, H1 advances more inclusive trials. H1’s diversity insights are crucial for creating better designs, enabling you to identify diverse investigators, patient populations, and leading trial sites—all from a single platform.
Reflecting on the Journey to Becoming a Health Equity and Diversity Champion
In the interview, Perez concluded his thoughts on the subjects, reflecting on his extensive career. He emphasized that the future of diversity in healthcare is the shared responsibility of both sponsors and CROs, who must actively drive change. Supported by the right technology, their joint efforts can significantly empower clinical research.
His guiding principle is the fundamental truth of healing, a mission that has driven his career and shaped his approach to healthcare. He believes that healing should be the ultimate goal, accessible to all rather than limited to the privileged few who can afford care within specific institutions. Perez envisions a future where barriers to equity gradually disappear, making comprehensive and fair healthcare a reality for everyone.
Tune into the entire conversation with Perez here.
Learn more about how H1’s data insights can help.
