Why More Data Hasn’t Improved Clinical Trial Enrollment

More Information, Same Enrollment Challenges
In clinical trial feasibility, we face a counterintuitive reality: despite unprecedented access to healthcare professional data, identifying the right investigators and predicting enrollment success has become increasingly challenging.
This paradox, highlighted in a recent Life Sciences DNA podcast featuring Ariel Katz, co-founder and CEO of H1, in conversation with Dr. Amar Drawid, an industry veteran and AI expert, merits deeper exploration for clinical operations professionals seeking to optimize site selection and accelerate patient recruitment. Their discussion on “Finding the Right Healthcare Providers from Clinical Trials to War Zones” offers valuable insights into this complex challenge.
Why More Information Hasn’t Improved Trial Performance
“It’s a beast, you know, it’s not getting any easier. You would think with AI it would get easier, but it’s actually creating somewhat more noise,” Katz observed during the podcast. Despite the explosion of available data, he noted that “the process of finding the right PI and the right site for a study hasn’t improved – we’re not seeing higher enrollment rates today than we were four years ago.”
This observation captures the central dilemma facing clinical operations teams today: The proliferation of data sources—from publications and clinical trials to social media activity and prescribing patterns—has created an information environment that’s simultaneously richer and more difficult to navigate.
What was once a relatively straightforward process of identifying investigators through professional networks and publication records has evolved into a multi-dimensional analysis challenge. The sheer volume of information available about healthcare professionals worldwide has paradoxically made it harder to determine who will actually deliver results for a specific trial.
Perhaps no aspect of this challenge is more critical—or more elusive—than accurately predicting which physicians will successfully enroll patients. As Katz bluntly stated, “Predicting a physician’s enrollment rate on an oncology clinical study, it’s a beast of a problem.”
Let’s examine these factors in detail.
The Multi-Dimensional Enrollment Challenge
Patient Population Access
The fundamental question is whether a physician has access to patients who meet increasingly complex inclusion/exclusion criteria. As Katz noted, “You have to find the doctor that meets this perfect identity painting of a patient, which is very difficult to do.” This challenge is particularly acute outside the United States, where granular patient population data may be limited or nonexistent.
Site Capabilities
Beyond the physician’s patient population, feasibility teams must assess whether “the place that the physician works has the capabilities and the facilities to do a clinical trial.” This includes research staff availability, equipment, and infrastructure—factors that often aren’t captured in standardized databases.
Investigator Motivation
The human element remains critical. As Katz candidly explained, sponsor relationships matter: “I work at Merck, do they like Pfizer? I work at Pfizer, do they like Merck?” Investigator preferences and motivations significantly impact enrollment success but are rarely systematically tracked.
Historical Performance
Past enrollment success remains one of the strongest predictors of future performance, yet comprehensive historical data across sponsors is difficult to obtain and normalize.
"Knowing where a doctor works, not that hard. Knowing how to predict a physician’s enrollment rate on an oncology clinical study, it’s a beast of a problem."
Moving Beyond Master Data
The podcast highlighted an important distinction between basic “master data” about healthcare providers and the deeper, more valuable information needed for effective trial planning:
“Master data is solved and it’s easier and it’s cheaper,” Katz explained. “Knowing where a doctor works, not that hard. Knowing how to predict a physician’s enrollment rate on an oncology clinical study, it’s a beast of a problem.”
This distinction helps explain why traditional approaches to feasibility often fall short. Clinical operations teams need to look beyond the master data (locations, specialties, contact information) to understand the factors that truly drive enrollment success.
The Data Integration Challenge
What emerges from the podcast is a clear picture of the true challenge: it’s not just having more data, but having the right data, properly integrated and contextualized. This is where comprehensive solutions like H1 come into play.
The key to successful investigator selection lies in integrating multiple data dimensions:
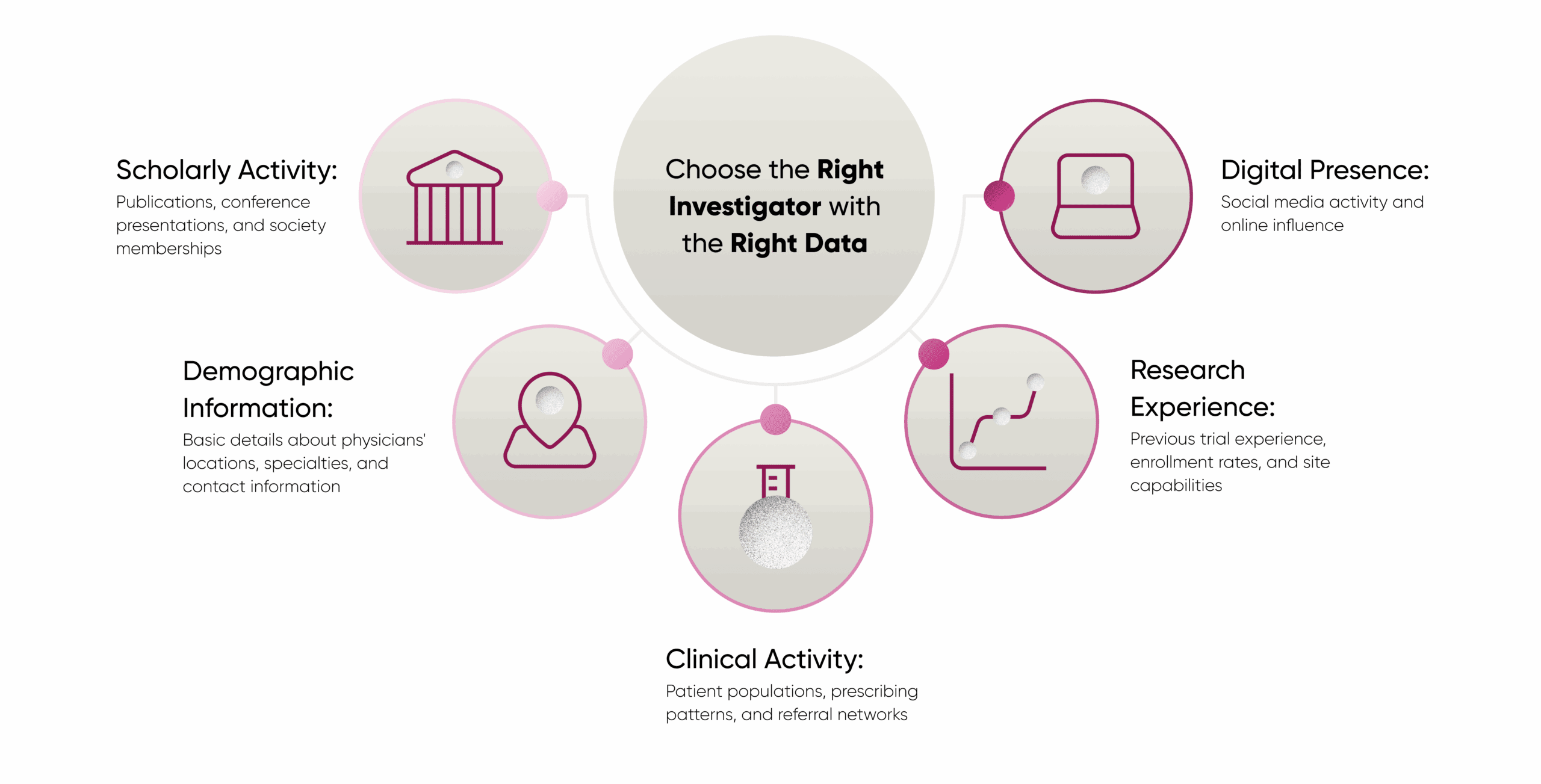
When these diverse data streams are properly integrated into a comprehensive knowledge graph—as Katz described H1’s approach—clinical operations teams can gain a much more nuanced understanding of which investigators will likely succeed with a particular protocol.
A knowledge graph, as Katz explained, connects healthcare professionals to their various attributes and to each other, creating a network of relationships that helps identify the most promising investigators. “We have a knowledge graph of who doctors work with, who they publish scientific papers with, who they share patients with, who they refer patients to, who they go to medical school with…” noted Katz, highlighting how these interconnections provide crucial context beyond isolated data points.
Practical Implications for Clinical Operations Teams
For clinical trial feasibility professionals, this data integration challenge has several practical implications:
1. Seek Comprehensive Data Solutions
Rather than working with siloed data sources, look for platforms that integrate multiple dimensions of healthcare professional information.
2. Focus on Relationship Data
Understanding the networks and relationships between physicians can provide crucial insights into referral patterns and influence.
3. Prioritize Historical Performance
Previous enrollment success in similar trials remains one of the strongest predictors of future performance.
4. Leverage Diversity Data
Information about the demographic composition of physicians’ patient populations can help meet increasingly important diversity requirements in clinical trials.
Conclusion
The paradox of clinical trial feasibility in today’s data-rich environment is that more information hasn’t necessarily made investigator selection easier. As data volumes grow and trial protocols become more complex, the challenge is no longer data scarcity but data integration and contextualization.
Comprehensive healthcare professional data platforms, such as H1, address this challenge by aggregating and connecting multiple data dimensions to create a more complete picture of potential investigators. By moving beyond basic master data to a more nuanced understanding of the factors that truly drive enrollment success, clinical operations teams can make more informed site selection decisions and ultimately accelerate the clinical trial process.
