New Report from H1 Reveals Disappointing State of Diversity, Equity, and Inclusion in Clinical Trials

Data highlights diversity gaps in U.S. clinical trials and opportunities for improvement moving forward.
NEW YORK, JULY 18, 2023 — H1, the leading source of truth for global HCP, clinical, scientific, and research information, today released an eye-opening report titled “2023 Industry Report: The State of Diversity and Equity in Clinical Trials.”
The Consolidated Appropriations Act of 2023, which requires drug and device sponsors to submit diversity action plans with their clinical trial data, has elevated the importance of inclusive clinical trials from an aspiration to a legal mandate. However, despite decades of government efforts and recent commitments from life sciences companies, designing diverse clinical trials and recruiting representative patients remains a significant challenge.
This report, based on U.S. Census data from 2020 and claims data within the H1 platform over the past two years, sheds light on the challenges and shortcomings of current approaches, which largely rely on census data only, and introduces a new strategy that leverages data-informed primary investigator (PI) and site selection to achieve meaningful diversity and equity in clinical research.
Key findings include:
- Despite the commitment of the FDA and life sciences companies and requirements for draft guidance submissions, diversity goals in clinical trials are not being met due to flawed approaches and discrepancies in standards around diversity metrics.
- Certain patient populations, including racial and ethnic minorities, adolescents and young adults, older adults, women, low-income individuals, and individuals from rural communities, remain consistently underrepresented in clinical trials due to lack of access.
- There is a disconnect between census and claims data, actual patient populations, and trial locations. For example, in the area of Grand Rapids, Michigan, census data shows a significant number of Black patients but a low number of claims, suggesting patients may seek treatment elsewhere.
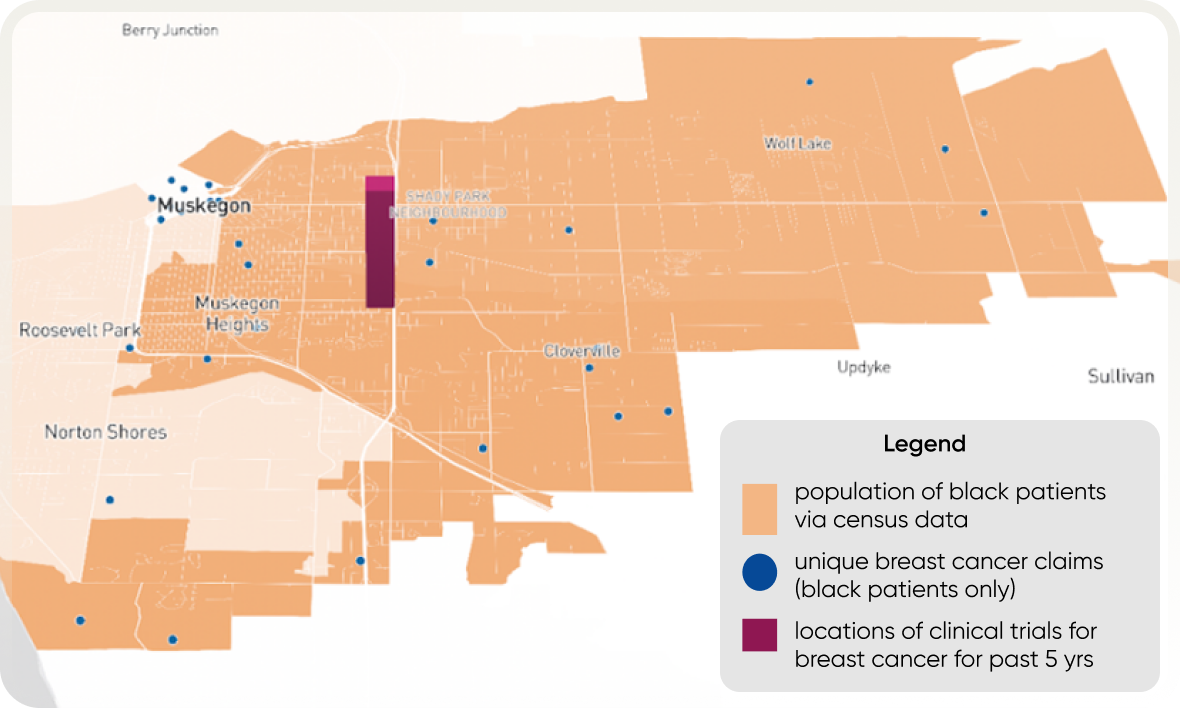
- A new approach to clinical trials that goes beyond census data to gain a deeper understanding of patient populations and their specific barriers to trial participation is recommended.
- Consideration of potential primary investigators’ (PI) race, languages spoken, patient populations, clinical trial experience, affiliations, and publications can assist in identifying the right doctor and site for diverse patient enrollment.
- In addition to PI and site selection, other barriers to participation must be addressed to ensure diverse representation in clinical trials.
“As an industry, we all agree that inclusivity and proper representation in clinical trials are not optional,” said Ariel Katz, CEO, H1. “Our report highlights the distance between intentions and change. Companies have to go beyond surface-level demographic data to understand impacted patient populations and the hurdles they face in participating in trials.”
Click here to download a complimentary copy of the report.
About H1
H1 connects the world to the right doctors and is the leading source of truth for global HCP, clinical, scientific, and research information. Leveraging next-gen analytics and AI, H1’s enterprise solutions democratize access to HCP data, diversity insights and groundbreaking research for life sciences, academic medical institutions, health systems, and payers. The H1 platform fuels a robust product suite that helps support the advancement of innovative and inclusive medicine. Today, more than 250 customers trust H1 to keep them current with the latest information on HCPs everywhere including their clinical and scholarly work and spheres of influence, connect them with the right thought and treatment leaders digitally and face-to-face, guide their strategies to increase adherence to evidence-based medicine, inform inclusive clinical trial design, provide access to groundbreaking science, and accelerate time to market. Learn more at https://www.h1.local.
Media Contact:
Kristina McConnell
Marketing Director, H1
[email protected]
