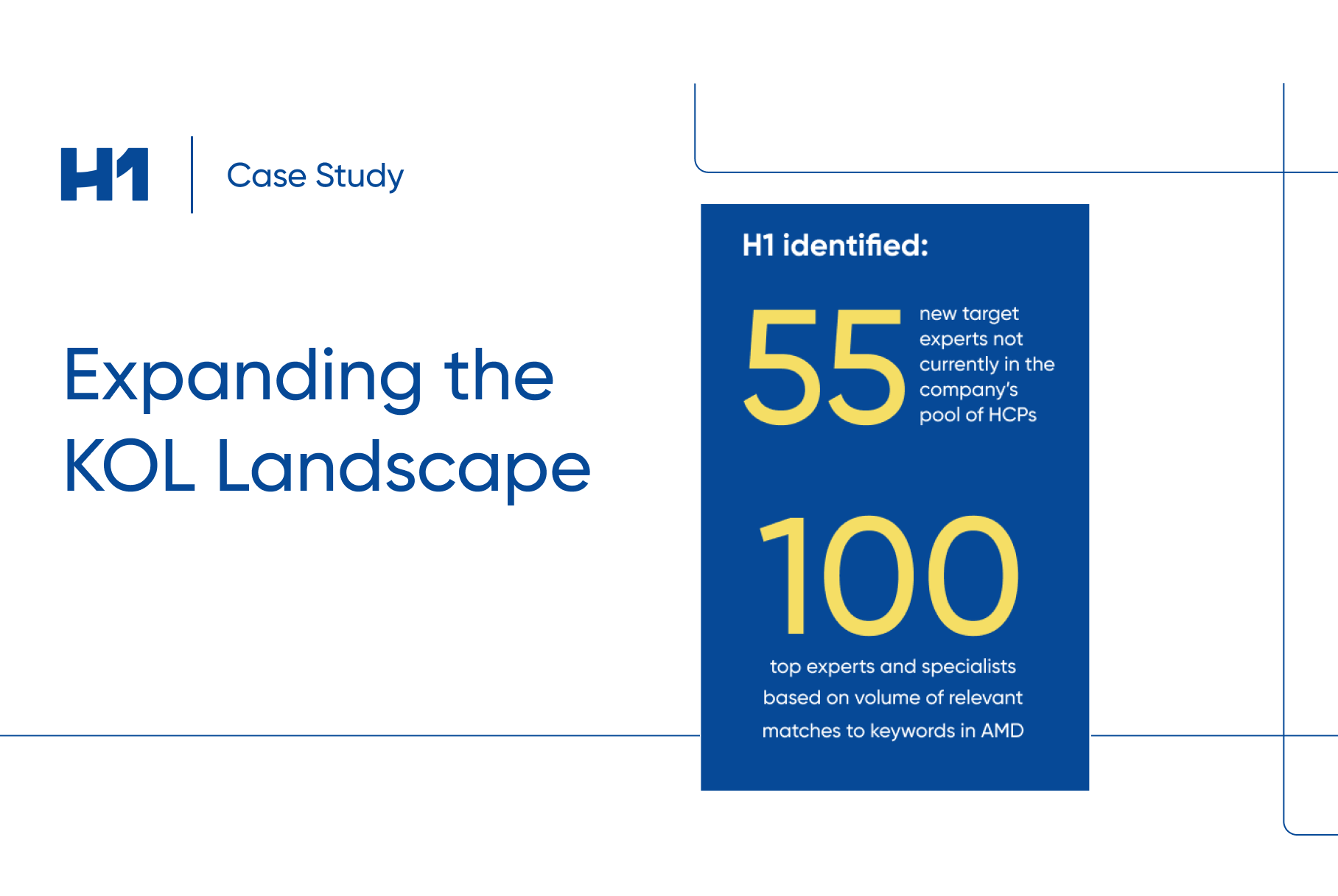
2023 Report: The State of Patient Representation in Clinical Trials
Representation in clinical trials is no longer an ambitious target but a legal mandate.
Last year the Food and Drug Omnibus Reform Act of 2022 brought a major shift to clinical trial development by mandating drug sponsors to submit their diversity action plans for phase 3 or other pivotal clinical trials. Non-compliance can lead to drastic consequences like potential rejection of drug applications, causing significant delays and financial burdens.
As patient representation now takes center stage in clinical trial development, our latest industry report, The State of Patient Representation in Clinical Trials is your resource to enhance trial inclusion and meet FDA requirements and understand some of the challenges and opportunities that have surfaced during national efforts to improve patient representation in trials.
In the report we explore:
- A deep dive into why census-based site selection fails to ensure patient representation.
- Insights into meeting FDA regulations for successful drug application approval.
- A data-driven site selection strategy with proven results from industry leaders.
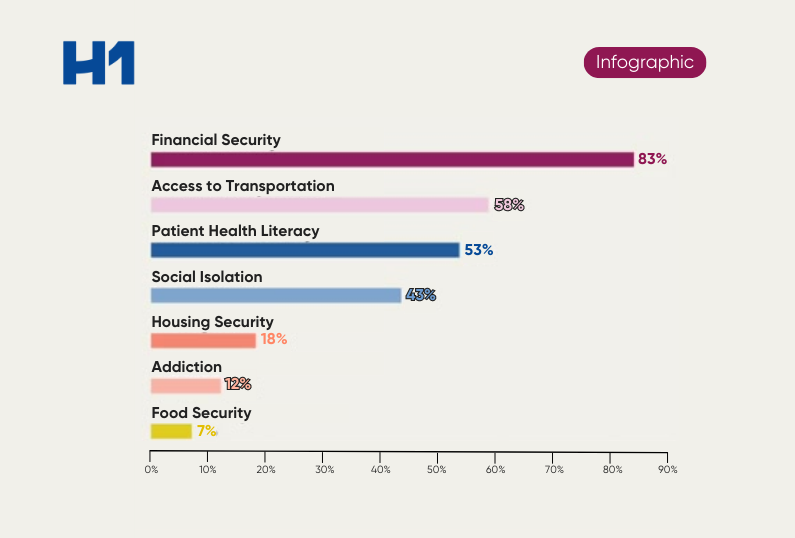
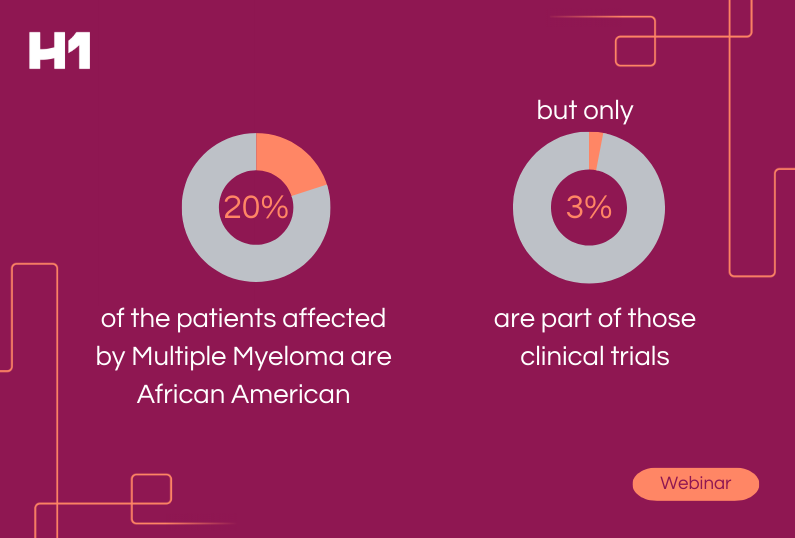
- Real-world data evidence from H1’s platform highlighting disparities in trial access for vulnerable populations specific to oncology.
Download the industry report below: