3 Common Questions Related to Clinical Trial Performance Data

Over the past decade, the cost and time impact to bring drugs to market have been increasing. With the recent trends in healthcare (more inclusion for both patients as well as physicians) and the economy (recession and hyperinflation), this is not a trend we expect will decrease any time soon.
One of the few elements industry players can control is better use of the data elements captured and available to them. Clinical trial performance knowledge on physicians and research sites is one of these elements. H1 is pioneering in the area of clinical trial performance data integration and use and regularly advises customers in 3 key areas.
What Is Performance Data and How Do You Benchmark It?
Clinical trial performance data is the collection of data elements which represent how well clinical studies have done in the past. This data is captured by the trial sponsors and usually collected in a Clinical Trial Management System (CTMS) or a similar data capturing environment. Performance data is usually captured on a site and physician level but can be rolled up on a country, geography, and global trial level.
This data contains details related to trial timing, site opening, patient enrollment dates, expected enrollment vs. actual enrollment, patient screening and patient drop-out. The specific level of detail captured is dependent on the data depth expected by the trial sponsor and can be very different between sponsors and systems.
If the performance data of multiple trials is combined, the data can also be rolled up on other levels such as the indication or Therapeutic Area level, as well as based on trial phase, trial type or any other trial related indicator. If the trial performance data is combined, especially trial performance data from multiple trial sponsors after it has been anonymized, it is possible to create industry general performance benchmarks on a multitude of levels (physician, site, indication, phase, country, geography, TA, …).
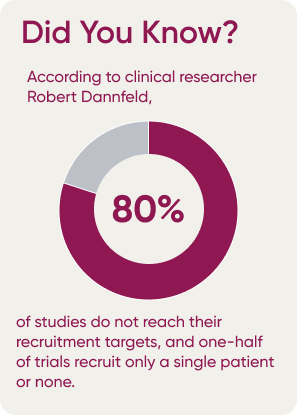
How Can Performance Data Make a Difference?
The 3 main benefits realized through the use of benchmarked performance data are accurate predictive enrollment models, comparing trial performance of physicians and sites against the rest of industry, and assessing performance of potential new physicians and sites, based on the experience other industry partners have had with them.
Benchmarked performance data provides access to selection insights and creates accurate enrollment forecasting models for upcoming clinical studies based on a combination of historical information and current input data. The model can take average performance values of individual PIs and sites into account to get more realistic estimates on how long it would take to recruit and evaluate a certain type and number of patients, as well as how successful they were historically in keeping these patients engaged, based on similar types of studies.
Comparing the performance of physicians and research from internal knowledge against performance for other industry partners can be a very valuable source of information. Especially in combination with other data points such as the competitive landscape or clinical payment details, it might highlight competitive impact challenges, sponsor affinity or compensation and support differences.
Starting to work with a new site or investigator is like taking a leap of faith, especially if their historical trial performance is unknown. Having insight into how well they have performed on similar trial designs for other industry sponsors can provide an extra layer of confidence required to take that step. Access to the performance data can and help learn from the choices and success of others, as well as prevent mistakes getting repeated.
What Are the Challenges with Trial Performance Data?
One of the main challenges with trial performance data is the inability to access it. The data is owned by the study sponsor and even within the sponsor company, is often inaccessible to most individuals who would benefit most from it. The data is unavailable to CROs and individual research sites and knowledge from CROs and research sites is often lost in translation.

H1 has solved for both issues time by creating a clinical trial performance data network of multiple parties who are involved in the clinical trial execution process, from pharma to CROs and research sites, and combining the data and knowledge directly from the source. The main benefit from this approach is the magnitude and depth of data, otherwise unavailable to anyone. As the data sponsor information is anonymized and secured before integration into the network, it becomes very reliable as an industry benchmark and much easier to access.
