Four Strategies for Preparing FDA DAPs

Bridging the Gap Between Pharmaceutical Development and Advancement of Therapeutics
In the race to bring new treatments and cures to market, clinical trial operations and feasibility teams often face a formidable challenge: ensuring that patient populations are accurately represented in their drug development studies.
To help address this issue in the United States, The Food and Drug Administration recently issued a new draft guidance to industry for developing plans to enroll more participants from underrepresented racial and ethnic populations in clinical trials in the United States. As drug and device companies prepare for these changes, they must be aware of the implications for trial site feasibility and success, trial recruitment and diversity, principal investigators, and other stakeholders.
The FDA’s new draft guidance on achieving greater diversity in clinical trials is meant only as a suggested framework for doing so. Regardless, this roadmap can serve as an invaluable resource for companies looking to bring treatments and cures reflective of real-world patient populations more quickly and effectively into patients hands.
Clinical trial operations teams should consider implementing a few key strategies outlined by the FDA. We’ll also explore how organizations can leverage technology to manage their progress toward meeting these goals.
In this blog post, we’ll discuss how to leverage the FDA’s guidance to increase both trial site feasibility and success, as well as improving trial recruitment and diversity efforts – ultimately bringing drugs to market that are reflective of real-world patient populations.
A Framework that Will Become a Standard
First, it’s important to understand the FDA’s diversity requirements. The 2023 omnibus spending requires drug and device companies to create comprehensive plans for ensuring clinical trial diversity in order to be approved by the FDA for new indications or new products.
This includes factors such as gender, race/ethnicity, and age. Companies must also provide information about how they plan to increase trial recruitment from underrepresented communities and a clear understanding of their current clinical trial population and identify areas where additional representation is needed. Here are a few strategies we use to help our clients at H1:
 Establish Clear Metrics for Patient and Site Selection
Establish Clear Metrics for Patient and Site Selection
Before getting started, it’s important to determine what level of racial/ethnic enrollment is desirable in your clinical trial population. This will guide the development of your plan and help ensure that it meets FDA standards. With clear metrics in place, PIs can ensure that their trials meet FDA standards.
 Leverage External Stakeholder & Data/Analytics
Leverage External Stakeholder & Data/Analytics
The FDA encourages industry to consider data from EHRs, existing cohorts or real-world studies when developing their plans, as well as to look for opportunities with existing data and data platforms like H1’s clinical solutions to leverage technology with access to rich data sets like claims.
This data can help teams discover sites that recruit diverse patients and perform well along with insights on all affiliated HCPs like patient demographics and their history as a treater, scholar, and trialist.
Additionally, the guidance suggests that drug companies consider partnering with researchers and organizations who have established relationships with diverse communities – such as patient advocacy groups, community-based organizations (CBOs) or local healthcare providers – to identify potential participants and build trust.
 Surface Institutions & Sites Where Patients Are Treated Today
Surface Institutions & Sites Where Patients Are Treated Today
Utilize real-world evidence when developing your plan – this will give you a better understanding of what works best at recruiting diverse participants for a given study protocol and geographic location.
Data analytics can provide useful insights into participant recruitment by leveraging comprehensive data that can provide detailed information about the demographics of participants in past studies as well as their responses to various treatments.
Institutional participation can be a challenge for trial recruitment teams. It can also be difficult for Principal Investigators to find available patients at institutions that have the appropriate diversity as well as identifying surface institutions where diverse patients can be treated. These strategies can help organizations ensure that their trials are not only compliant with regulatory standards but also more effective in meeting the needs of all patients.
 Monitor Progress & Make Adjustments
Monitor Progress & Make Adjustments
Make sure you’re regularly monitoring progress towards meeting your enrollment goals for each study site throughout the course of the trial – this will allow you to quickly adjust strategies if needed in order to improve site feasibility and success rates by increasing racial/ethnic representation among participants.
Organizations can also use technology to manage their progress toward meeting the FDA’s diversity requirements. Technology-enabled analytics can also enable organizations to monitor their progress over time and make adjustments as needed in order to meet their goals more effectively and efficiently.
Finally, it’s important for organizations to partner with stakeholders outside of the pharmaceutical industry who have expertise in increasing diversity and addressing health disparities in clinical trials. These partners can offer invaluable insight into how best to engage hard-to-reach populations and customize approaches based on local needs and challenges.
What’s Next
That said, it is critical to remember that there is no one-size-fits-all approach to achieving these objectives. Factors such as the size and complexity of the trial as well as the company’s culture and resources will play an important role in determining how best to achieve each of these five key strategies.
As drug and device companies await further action from the FDA on this issue, they should take steps now to ensure that they are ready when its regulations come into effect in 2023.
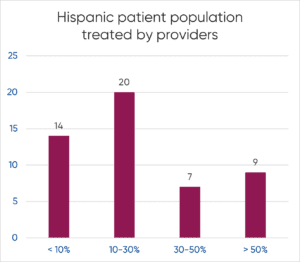
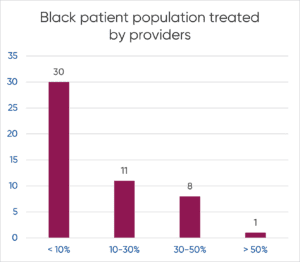
H1’s Patient Universe can help support companies with this requirement by providing reports and intelligence about the diversity breakdown of the patient population treated by principal investigators as well as diversity metrics of the providers themselves, which could indicate better identification and connection with the intended patient population targets.
Leveraging the five key strategies outlined above will go a long way in ensuring that companies are designing studies that are more likely to achieve regulatory approval but are also designing studies that reflect true research outcomes for all patients regardless of their race/ethnicity background.