
Maximizing Clinical Trial Success
Strategies for Optimal Site Selection and Feasibility Research in Novel Therapies and Geographies
According to the National Institutes of Health (NIH), clinical trial feasibility is a process of evaluating the possibility of conducting a particular clinical program / trial in a particular geographical region with the overall objective of optimum project completion in terms of timelines, targets and cost.
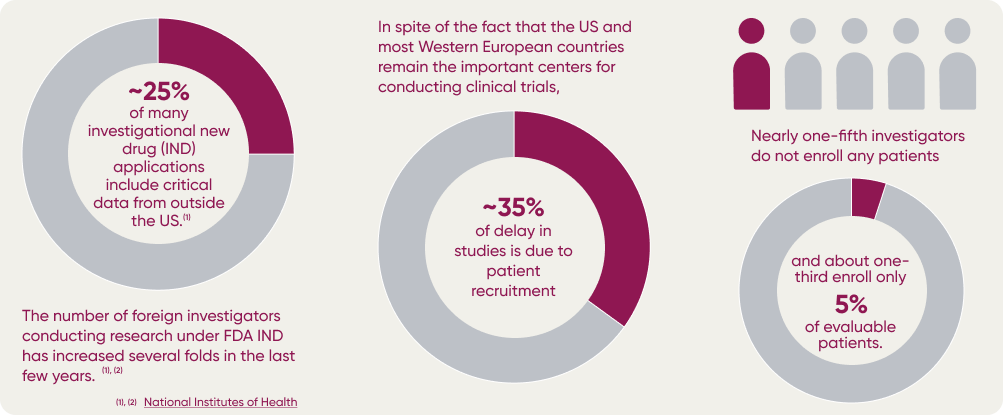
Clinical research teams in the pharma-biotech industry are under increasing pressure to set up and execute clinical trials quickly and efficiently. This is especially true for novel therapies, where time is of the essence. To maximize trial success, it’s essential that teams invest in effective site selection and feasibility research.
Assessing New Label Expansion Trial Sites: Data at the Indication Level
In this case study, we’ll highlight how one organization took a data-driven approach with H1’s clinical trial intelligence platform. H1 Clinical solutions helped the company find key strategies, data insights and analytics for optimizing the site feasibility process and ensuring successful clinical trials in novel therapies.
When evaluating prospective sites for a clinical trial, it’s important to consider the following factors: location (both in and outside of the U.S.), size and expertise of staff, patient demographics, infrastructure and access to specialized technology.
Data analytics and insights platforms like H1 can help streamline the process of evaluating and comparing sites by providing real-time access to site information. These tools can also be used to analyze data more efficiently, allowing investigators to make informed decisions quickly.
By taking advantage of these technologies, teams can acquire deeper insights into their prospective sites before committing resources or selecting a final location that fairly represents all of the needed factors for success.
Download the case study.



































































































