2024 Report: The State of Patient Representation in Clinical Trials
The road to improving patient access to healthcare starts here.
From the latest FDA guidelines to generative AI, H1’s newest report examines the state of patient representation in clinical trials to identify opportunities for pharma organizations to incorporate the latest developments, market trends, and strategic insights that are critical for enhancing clinical trial inclusion.
This informative report will prepare you to:
- Meet FDA regulations on patient representation for trial sponsors.
- Ensure patient representation by using Generative AI to identify sites, PIs, and patients.
- Set reasonable recruitment benchmarks within each indication based on patient burden.
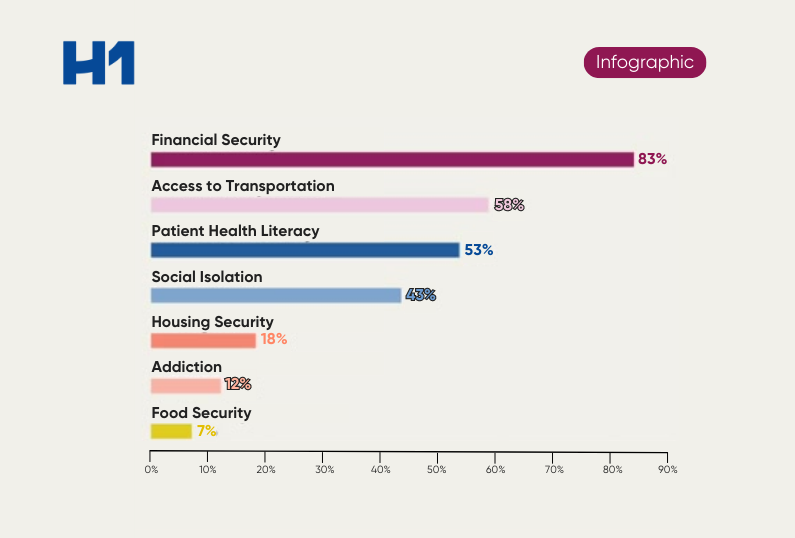
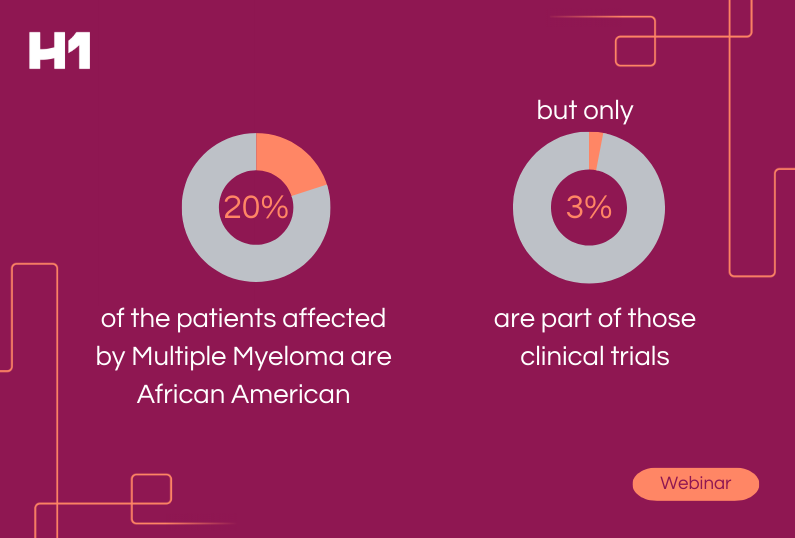
It’s time for pharmaceutical organizations to ensure research findings are applicable to the entire population to help foster improved health outcomes for all demographic groups.
Download the full report today to discover more about the state of patient representation in clinical trials.