Representation and Ethics: Moving the Needle on Real Change in Clinical Trials & FDA Guidance Prep
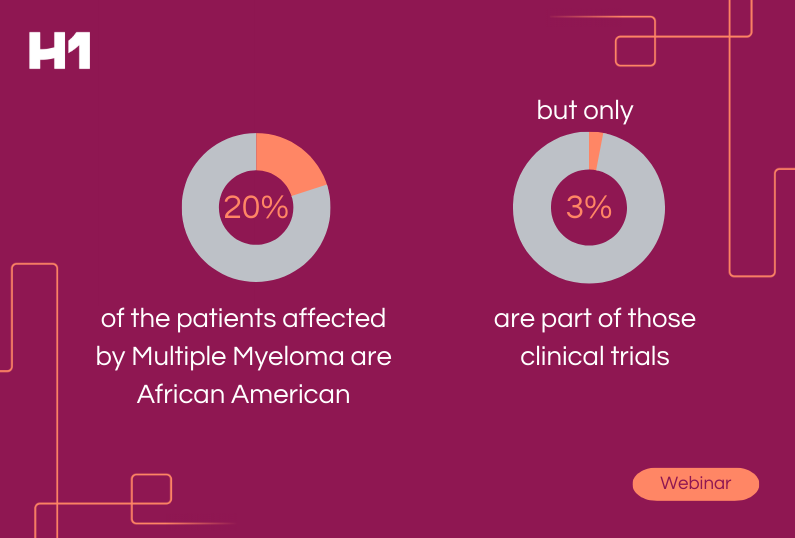
In a recent webinar with FirstWord, H1 explored current FDA guidance and discussed pending updates. Our panel went deep on operationalizing and measuring efforts that life sciences organizations and feasibility teams are making to improve enrollment of clinical trial participants from underrepresented racial and ethnic populations, many of whom are part of medically underserved communities. Other topics explored included:
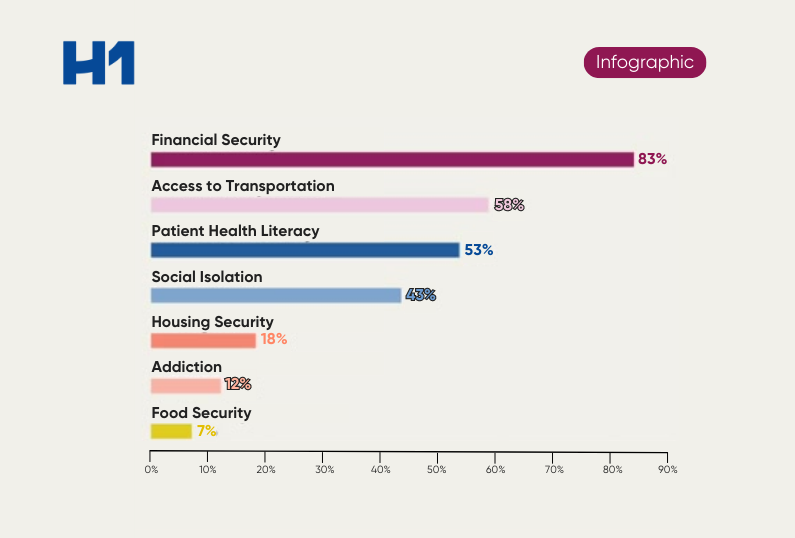
- The role of social determinants of health and other patient metrics in building representative trials.
- Building trust which plays a critical role in patient access and ensuring inclusive trials for drug development.
- Where and how to begin to build inclusive and tech-enabled trials – ideas for consideration.
Panelists:
- Ryan Brown, Regional Vice President, H1
- Tinaya Gray, Executive Director, Global Head Diversity in Clinical Trials, ICON
- William E. Fitzsimmons, Pharm.D., M.S., Board Member at CARER Group (Catalyzing Access to Research and Equity in Representation), a 501(c)(3) nonprofit, and Founder of Tutela
Watch the video below to learn more: