Accessing Underrepresented Patient Populations with Data
Despite recent guidelines from the Food & Drug Administration on reaching underserved patient populations for clinical trials, the industry as a whole is still severely lagging in making real progress. A recent issue of STAT news noted that in an FDA analysis between 2014 and 2021 fewer than 14% of drugs had clinical trial data regarding treatment benefits or side effects for Black patients.
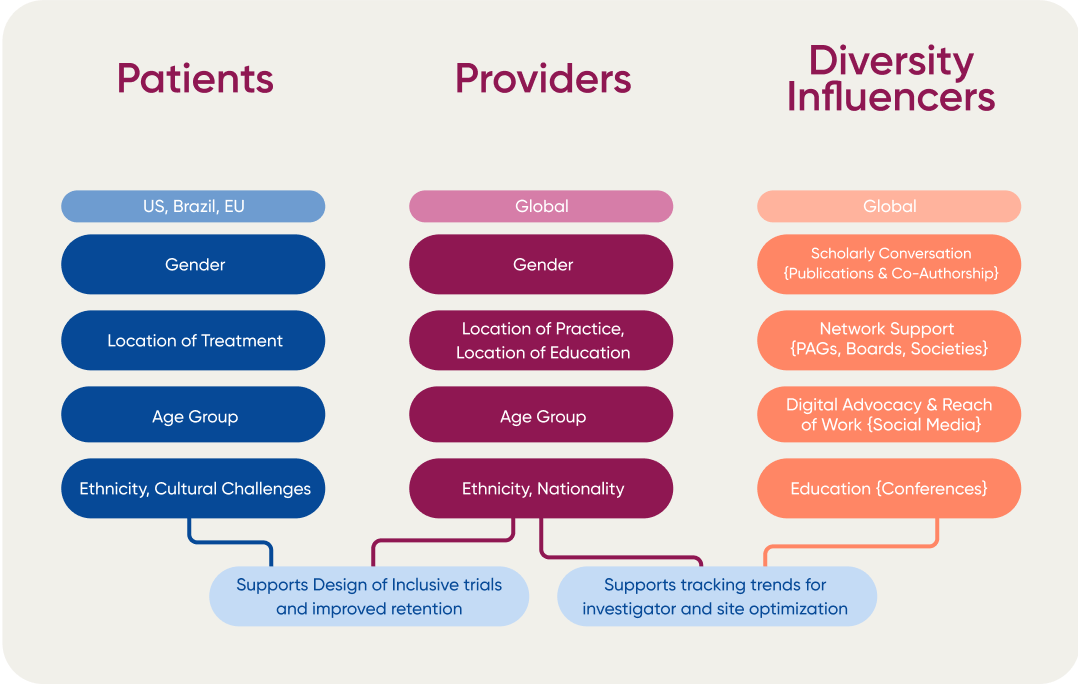
As a step towards broadening inclusion of both trial participants and site investigators, H1 provides in-depth analysis on clinical trials to provide an external resource for companies to be able to run more inclusive trials that represent the patient populations the drugs seek to help. With H1, clinical and medical teams are empowered to make impactful decisions by recognizing available research sites, potential trial investigators by assessing the global clinical and competitive landscape. Speeding up every step of the clinical process – from early phase clinical research all the way to drug launch – by supporting data-driven decision making based on continuously updated global insights.
Recently, an H1 client was struggling to find new potential Primary Investigators and clinical trial sites in specific regions. Their primary goal was to find HCPs with the right experience levels, and with access to desired patient populations in immunology for an upcoming clinical trial.
This client had already exhausted their internal resources, but did not have any information supporting their patient representation needs. They wanted to understand if any of their internally identified investigators and sites had access to a representative patient population, while also attempting to discover if there were other people or sites matching their criteria of which they were currently unaware.
How did they fare?
They wanted to understand if any of their internally identified investigators and sites had access to a representative patient population, while also attempting to discover if there were other people or sites matching their criteria of which they were currently unaware.
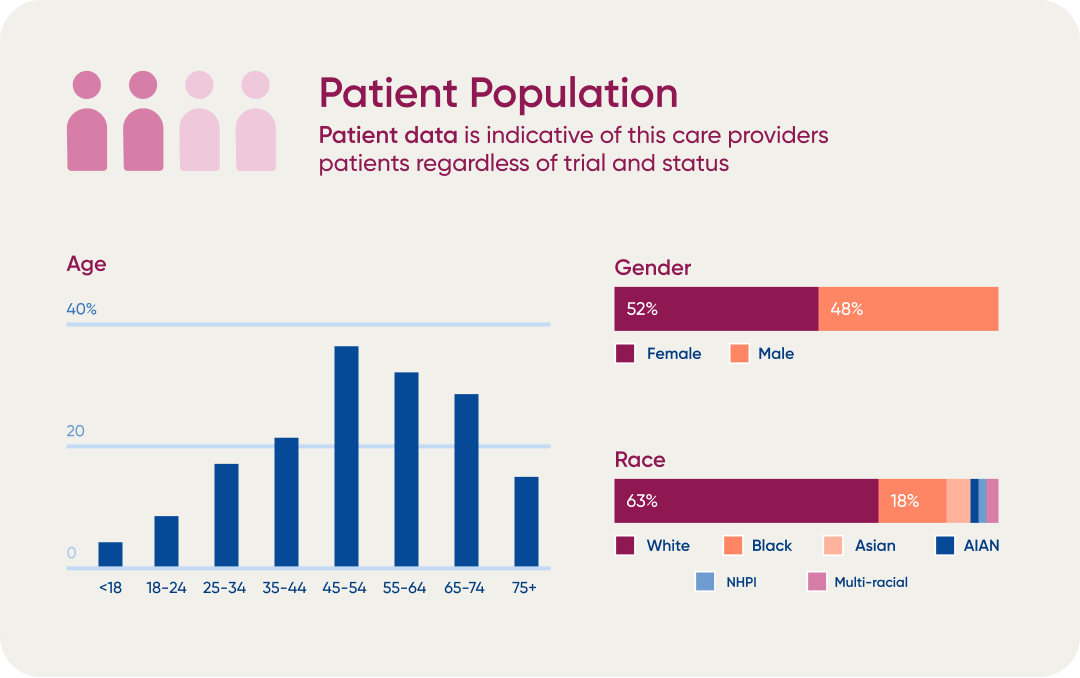
H1’s diversity data provided a market analysis resulting in a 60% increase in profiled people and institutions.
“H1 helped us recognize the institutions and HCPs that had the exact mix of experience we were looking to understand”
To help facilitate those needs, H1 partnered with Black In Immuno, an HCP network aimed at increasing the prevalence of HCPs of color within the Immunology space, to help provide self-identified D&I and experience information from providers. H1 then leveraged these relationships to increase the awareness and engagement of the client with these HCPs by 125% over their previous engagement.



































































































