
Patient Representation in Clinical Trials
Why Census Data Is Not Enough
Despite the commitment of the FDA and life sciences companies to increasing clinical trial inclusion, many study sponsors have continued to fall short of their goals because their approach to achieving patient representation is flawed.
One approach study sponsors commonly use is combining U.S. census data with claims data to identify areas within the country where both a particular disease of interest and a racial or ethnic minority coincide. For example, as of July 2018, U.S. census data shows that 79.1% of those in the City of Detroit are African American. If claims data also indicated there are a lot of breast cancer patients in the City of Detroit, then perhaps a study sponsor would try to establish a study center within Detroit to increase representation of African American women in a breast cancer clinical trial.

Just because a population lives in a particular area doesn’t mean that this population is treated at the nearest clinical center.

As a result, choosing a site based on local demographics may not result in the specific patient population enrollment one expects.

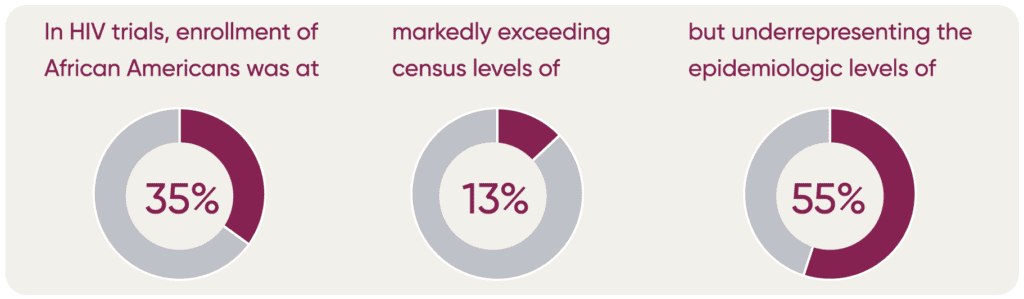
Moreover, U.S. Census Bureau data do not necessarily reflect the proportion of the population by ethnicity that may be impacted by a specific disease. A recent GSK study2 found that in four disease areas (asthma, COPD, HIV, and influenza) census data differed from the epidemiological data.
For example, U.S. Census Bureau data indicates that 13.4% of the total US population is Black/African American while the prevalence of asthma among this group in the U.S. is 17%, and the prevalence of COPD among this group is 7.1%. Similarly, census data indicates 18.5% of the population is Hispanic/ Latinx while the prevalence of different diseases among this group varies (asthma 14.4%; COPD 6.5%, HIV 35.7%; and influenza 10.4%).
The study also showed that GSK trial enrollment for each condition differed by race and ethnicity. Enrollment in clinical trials of African Americans for asthma (22.6%) exceeded both census (13.4%) and epidemiologic (17%) levels.
There are many other potential barriers to representative patient enrollment that you must address, including patient distrust or unawareness about the clinical research, inability to come in for regular clinical visits, and the overall high burden of trial participation. These problems with a census-based approach to addressing clinical trial representation necessitate a more sophisticated solution.
H1 empowers clinical study sponsors and feasibility teams by providing crucial information on PIs’ race, languages, patient population, trial experience, affiliations, and publications – beyond just census and claims data. Access these efforts effortlessly in H1 Clinical solutions to build equitable trials.
* Only 1 of 25 cancer drug developers fairly included minority patients over five-year window, BMJ analysis finds.
1 A doctor trained nurse practitioners to do colonoscopies. Critics say his research exploited Black patients.
2 GSK announces results from 17-year retrospective study on U.S. clinical trial diversity.


































































































