Best Practices for Enabling Accelerated and Inclusive Global Drug Development
In partnership with the Society for Clinical Research Sites (SCRS) H1 recently hosted an informative discussion on data-driven best practices for improving patient representation in trial sites.
We looked at the recently published recommended guidance from the FDA aimed at driving inclusive drug development and what pharmaceutical companies should be doing to prepare for future mandates.
H1 was also recently named a patron sponsor of the SCRS Diversity Awareness Program and is an SCRS Global Impact partner.
From exploring potential investigators for decentralized trials to protocol development and performance analysis, our experts shared best practices for getting started.
Highlights:
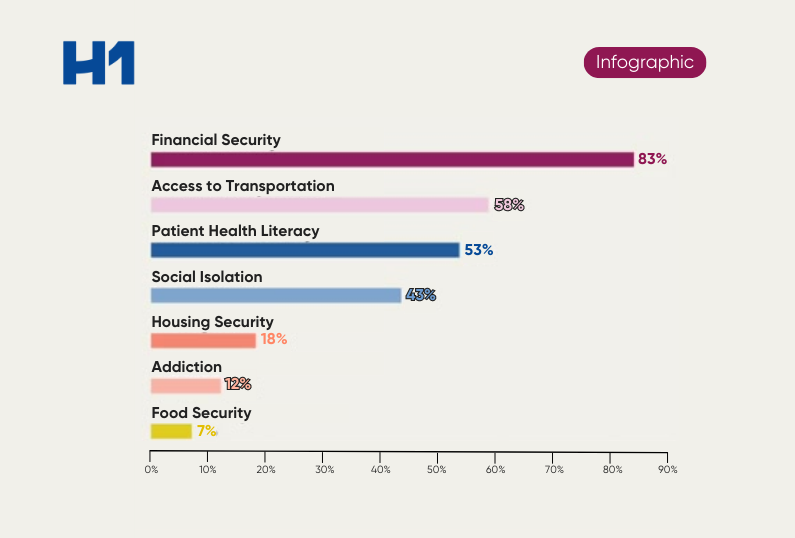
- Prescriptive best practices for data driven decision making to enhance representation in investigator profiles and patient populations
- How to find and understand inclusive key opinion leaders (KOLs) during product development
- Which key D&I data elements are critical to a sound strategy
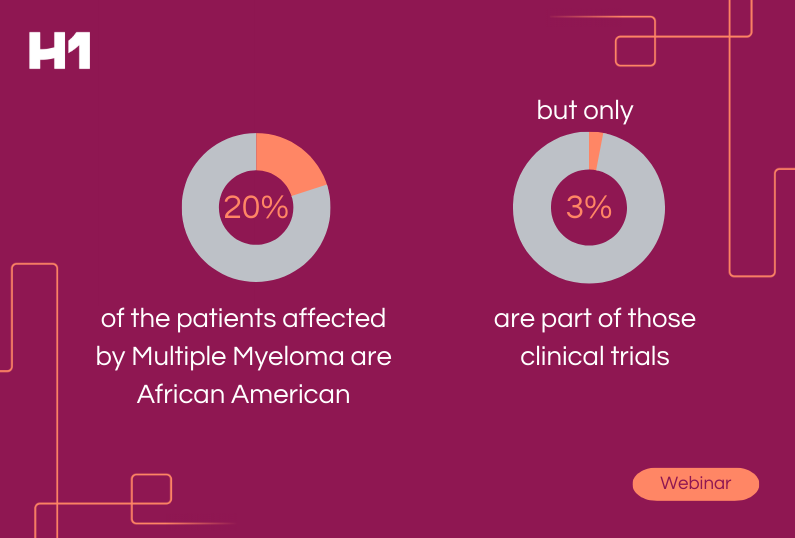
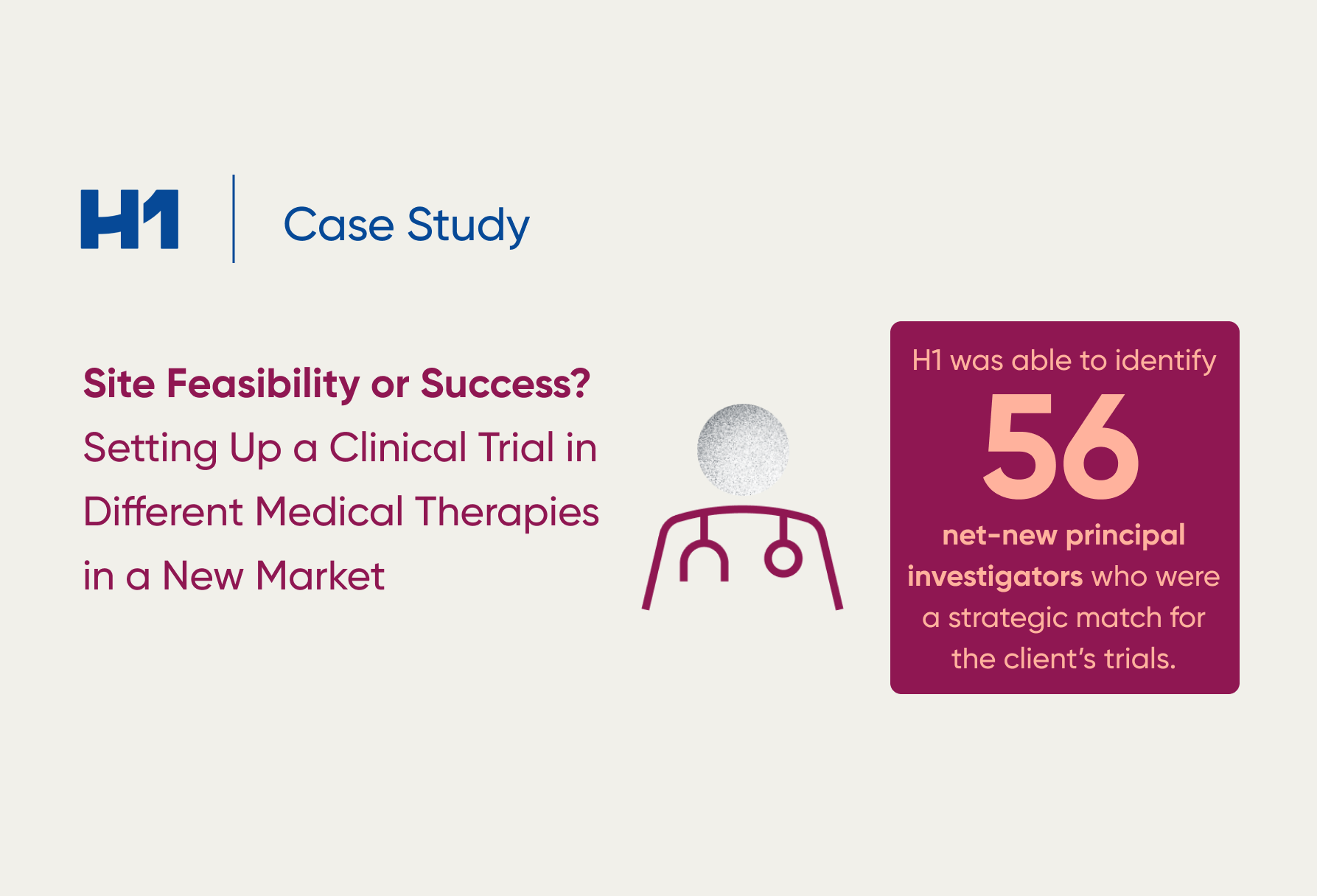
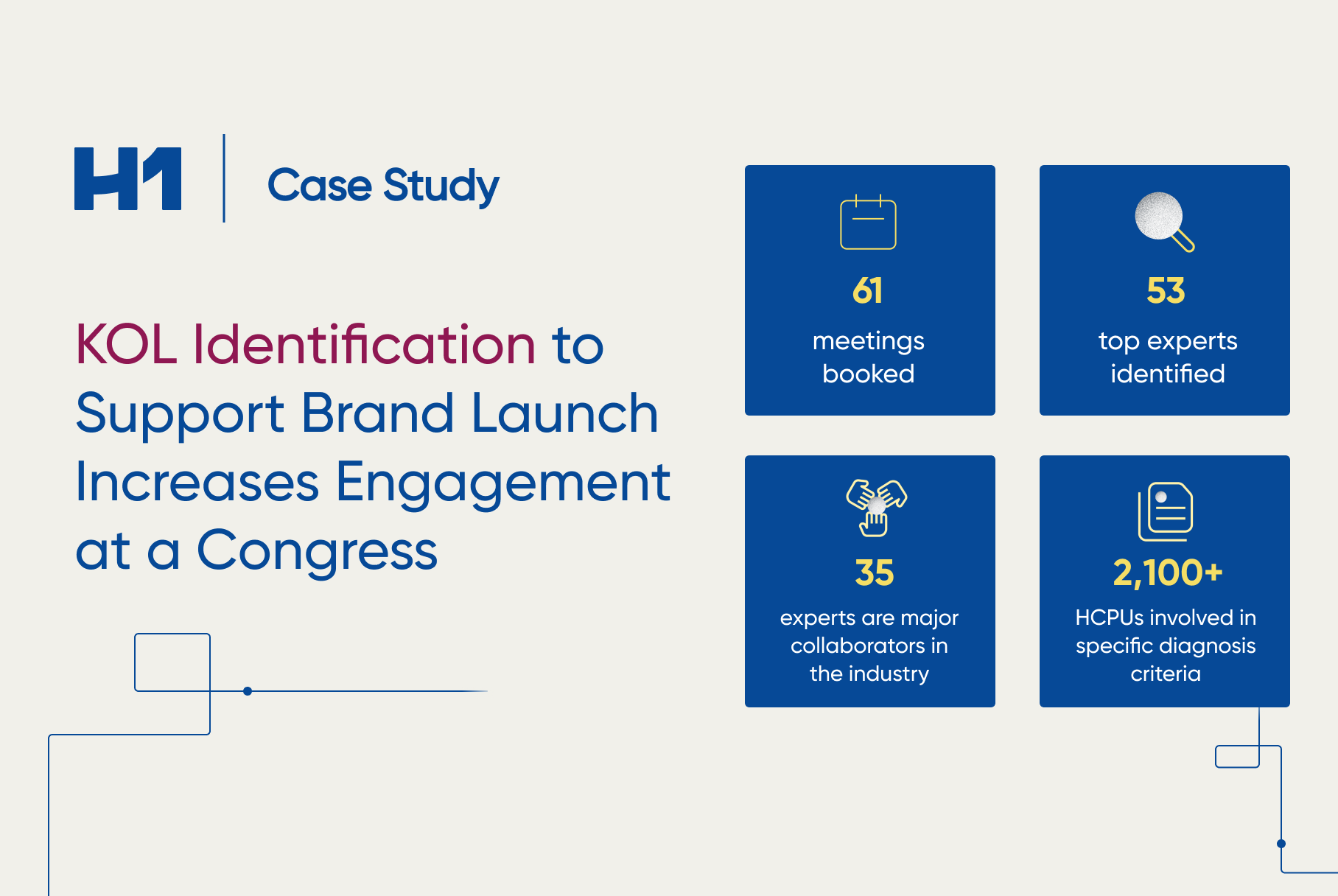
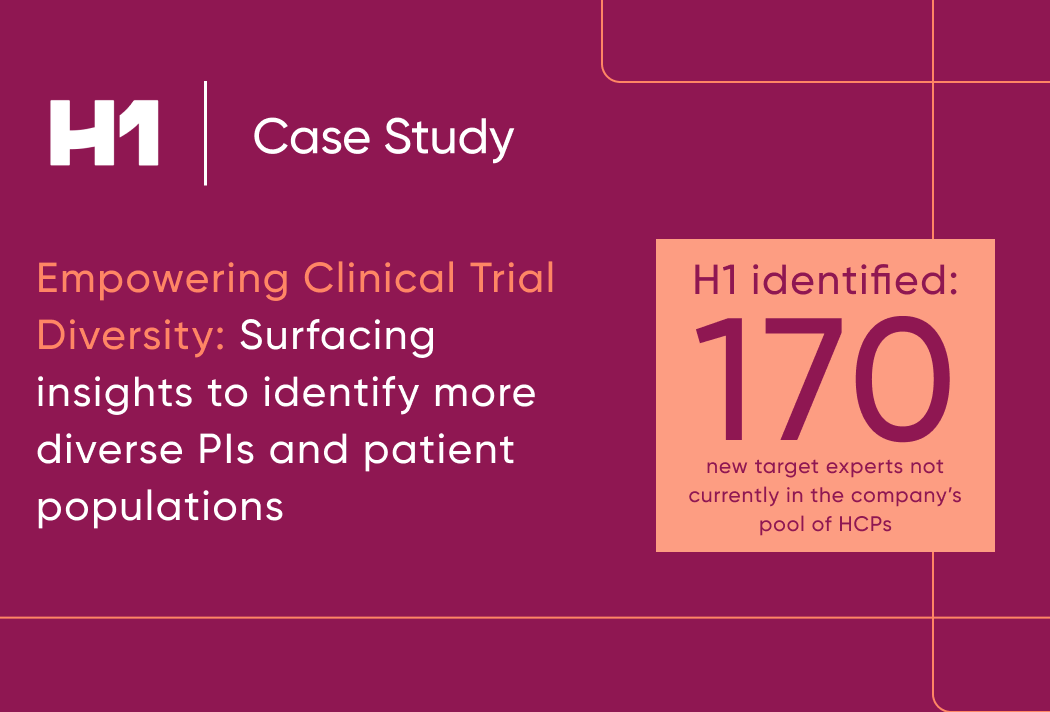
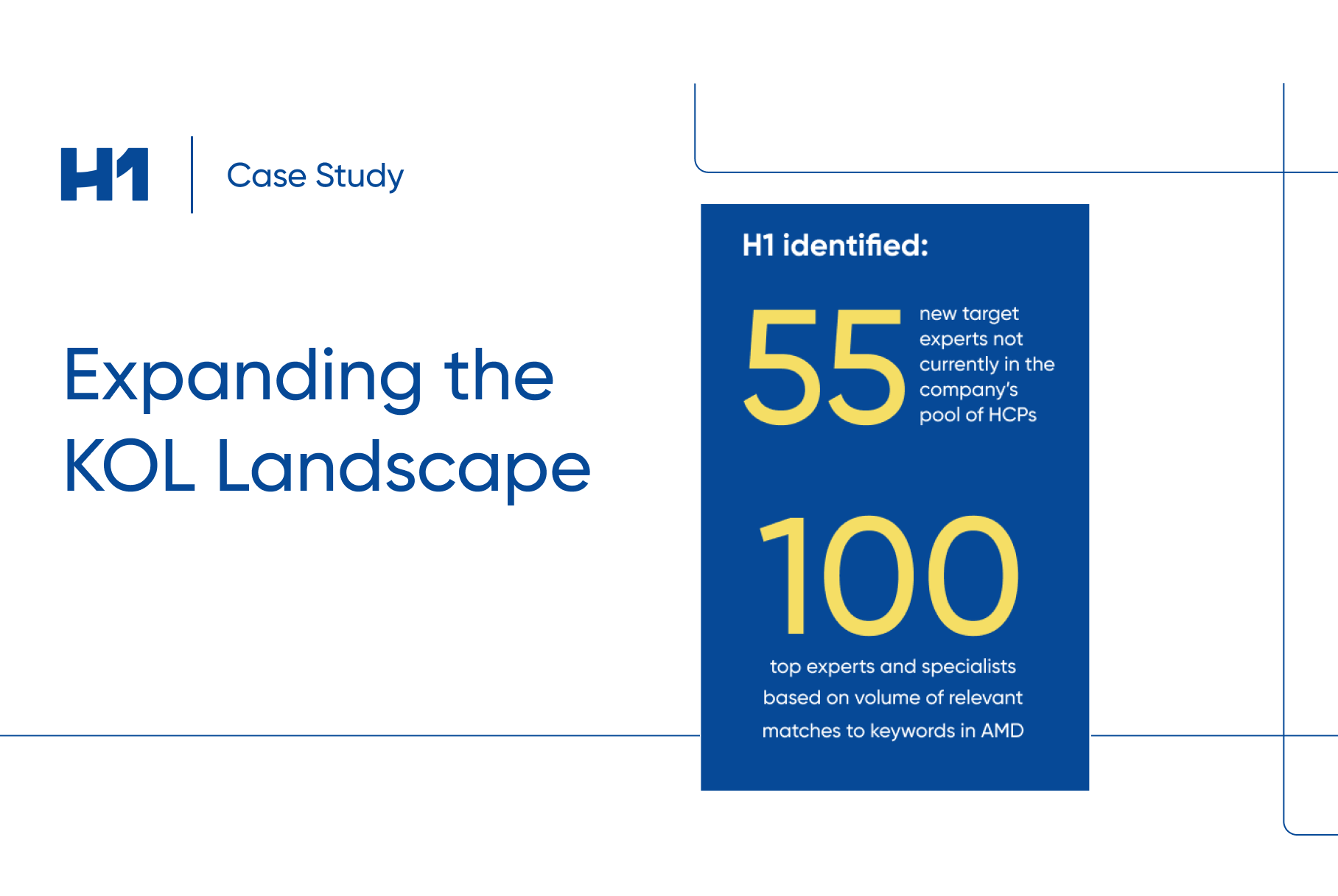
- Case study examples on how patient insights supported global pharma clients
Watch the webinar below: