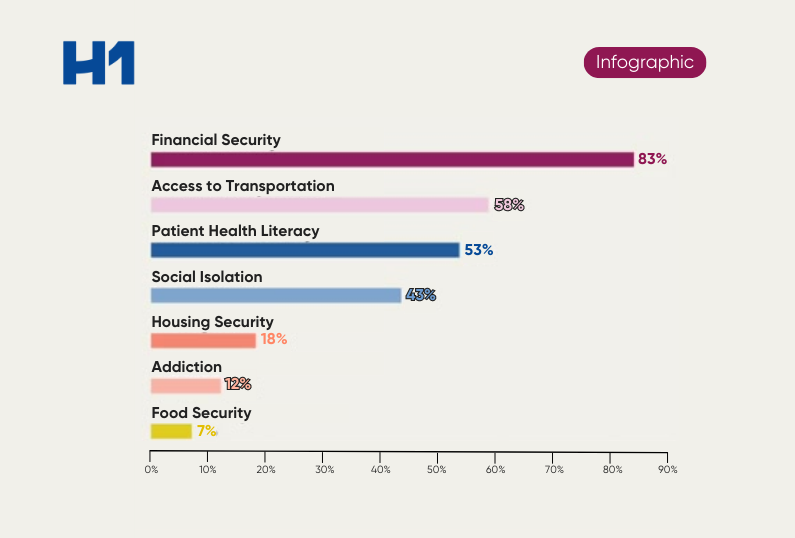
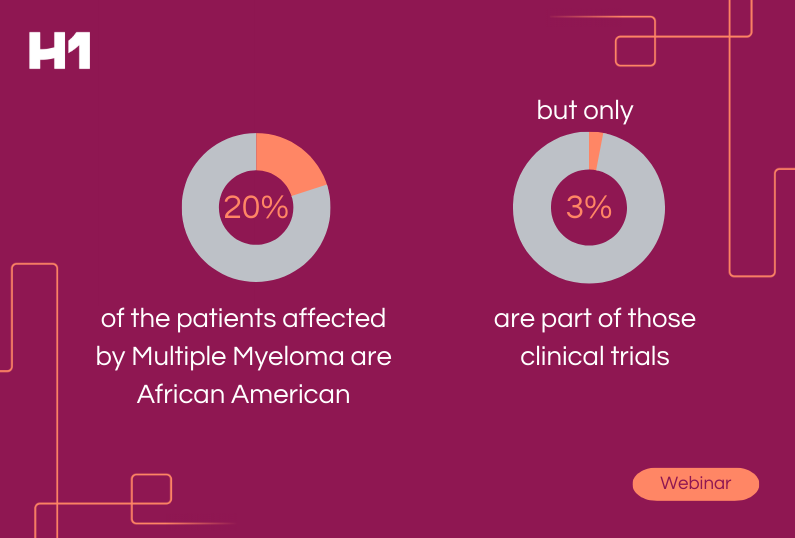
Representation and Ethics – AI and Drug Development
The Impact on Clinical Trials, Medical Affairs and Global Pharma
A white paper in partnership with H1 and FierceBiotech
The healthcare industry drives a critical balance between innovation and necessity. Within its dynamic landscape, a new emerging technology has the ability to revolutionize drug development, decision-making within medical affairs and subsequently, the effective treatment of millions of patients.
Artificial Intelligence (AI) and Machine Learning (ML) have ripened in recent years, emerging as powerful technological forces. Yet, an understanding of different types of AI — such as large language models (LLMs) and generative AI (GenAI) — still largely eludes the general public and much of the healthcare industry. But with a primary commitment to ethical practices and safeguards, AI promises to be a transformative technology within the biopharmaceutical sector, from streamlining drug developmental timelines and optimizing resource allocation to strategic planning and personalization within medical affairs teams.
Read now to learn:
- How AI can expeditiously sift through extensive data to provide faster analytics and predictions within early-stage research, generate insights on personalized medicine and even facilitate drug repurposing.
- Why these advancements can result in alleviated stresses on medical affairs teams, operational cost savings, improved trial feasibility and better patient outcomes.
- What ethical integration considerations remain paramount — including issues of privacy, transparency compliance and bias mitigation
AI is also reshaping healthcare in developmental processes and decision-making. This whitepaper will provide a wide-lens look at the state of AI in the biopharma industry and the best practices for integrating it into drug development and strategic planning while prioritizing ethical standards of equity and accessibility.
Download now.