Is Data the Answer to Inclusive Clinical Trials?
It does not fall on pharma alone to make sure that vulnerable populations have access to lifesaving medicine and therapies, but as FDA diversity action plans become required for clinical trials, pharma has to take a more active role in ensuring a more accessible healthcare system.
It starts with the HCPs you engage for pre-drug launch and marketing; it starts with the site investigators you recruit; it starts with the location and availability of the study.
In this panel, we discussed:
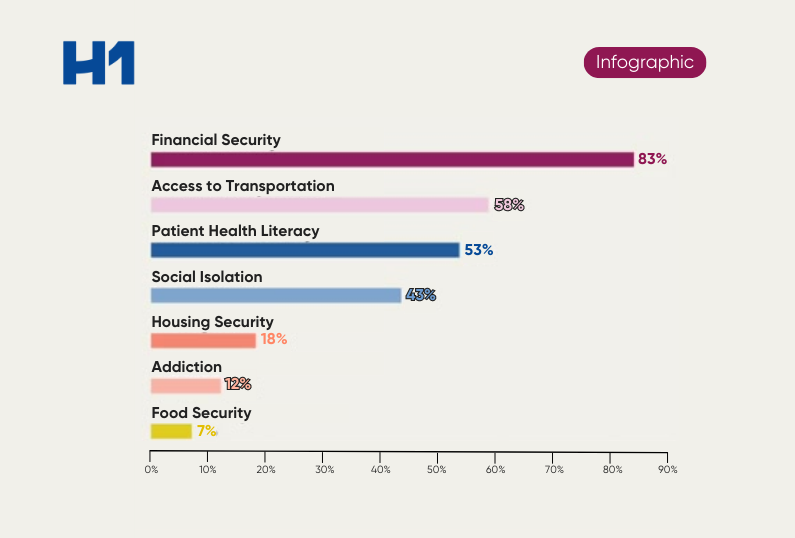
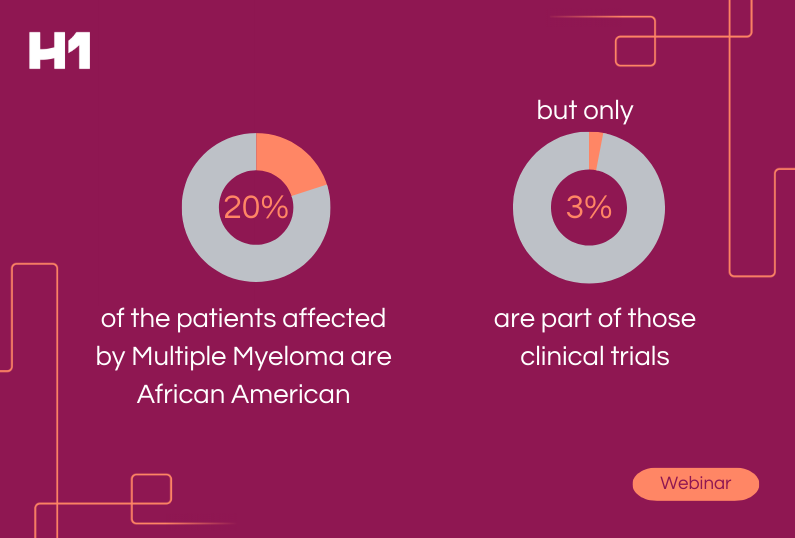
- Historically, what has been lacking in clinical trials when it comes to patient representation
- Why it took federal guidelines to begin to turn the conversation toward pharma vulnerable populations
- How big data can fuel representation in clinical trials and why the data matters
- What the ABC (and D’s) are of clinical trial access and inclusion
- Why geography and patient representation matter
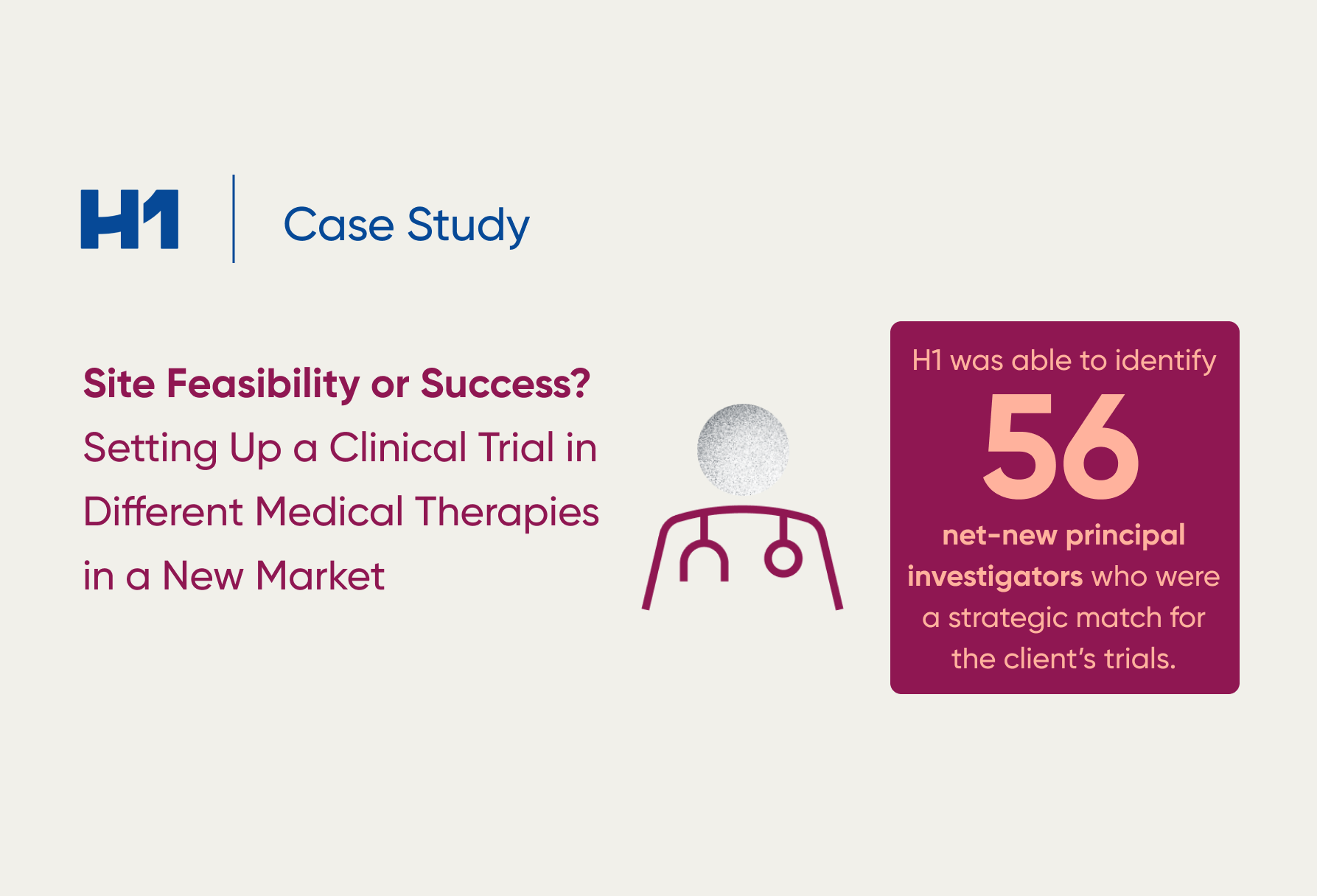
Watch the video. If you’d like to learn more about how to build equitable trials based on trial performance, feasibility, selection, PIs and patients, request a demo.