The Role of RWE in Clinical Trial Site Selection
Is data the new kryptonite for clinical trials?
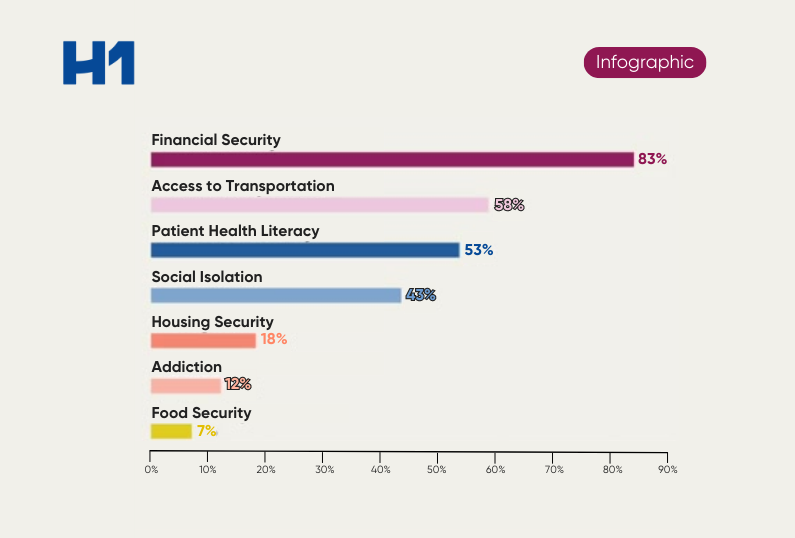
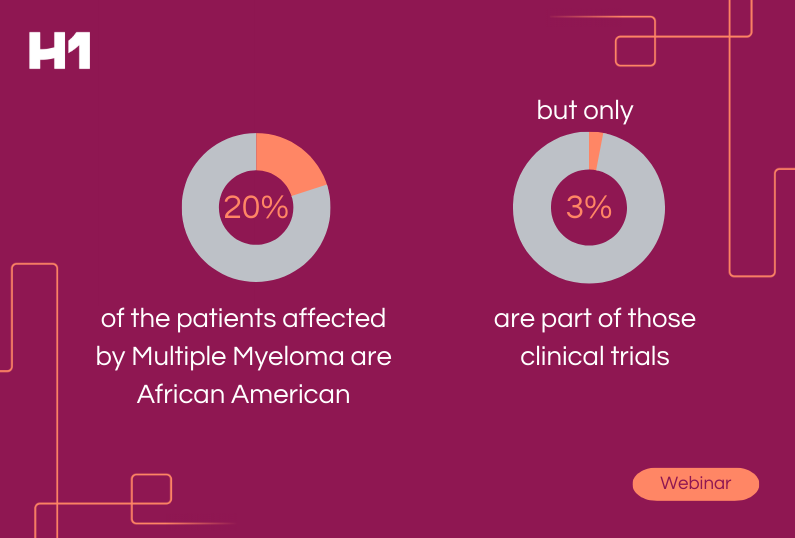
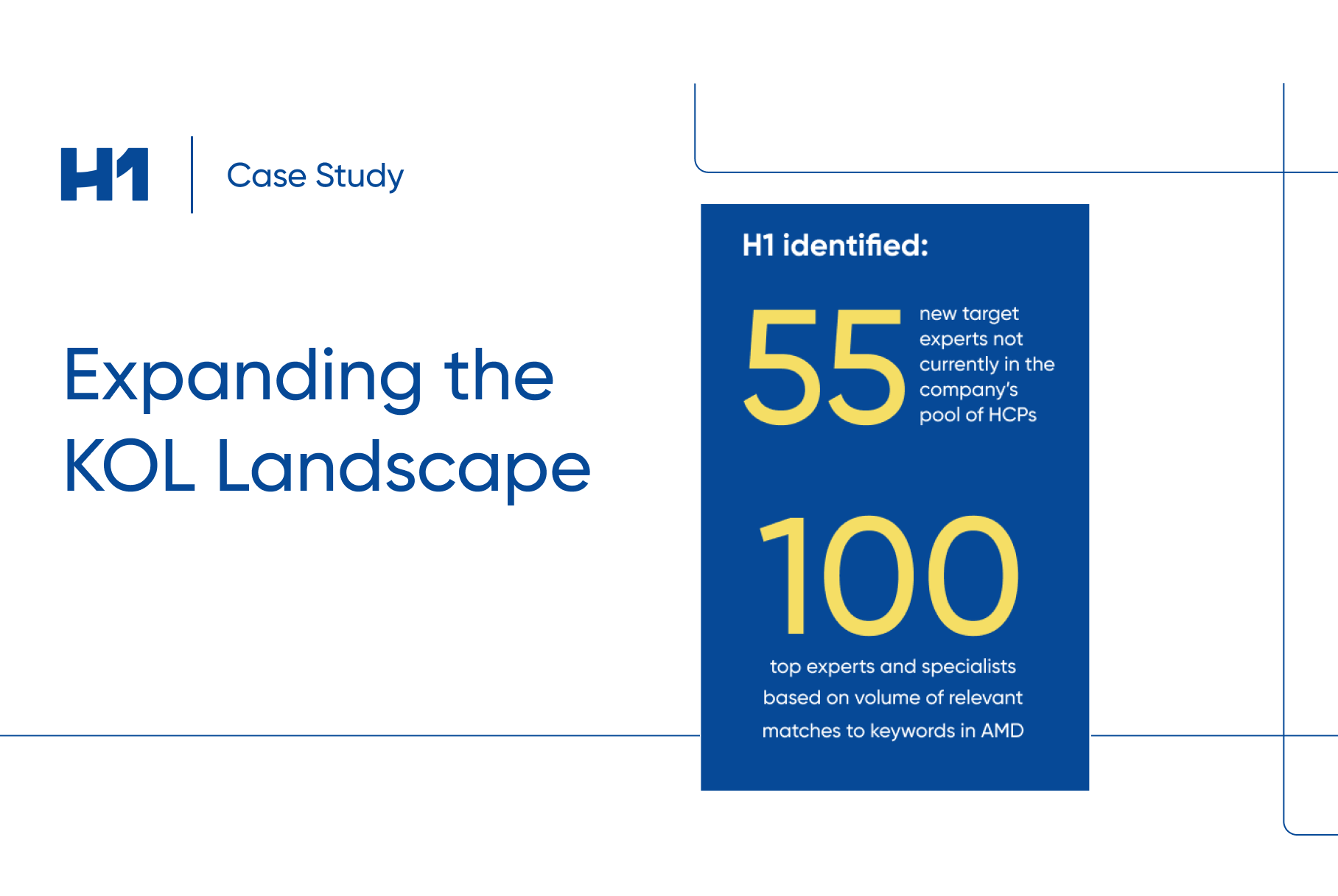
We all know that a successful clinical trial relies on the recruitment and retention of a diverse and equitable participant pool. But what happens when certain sites underperform, leading to costly delays and even study failures?
This is where real-world evidence (RWE) comes in. By tracking and analyzing data from past trials, RWE can provide valuable insights into which sites are most likely to meet recruitment and retention goals, follow study protocols, and generate high-quality data.
Download our white paper completed in partnership with FirstWord Pharma below: