
Building Trust and Inclusion for Equitable and Diverse Clinical Trials
Clinical trials drive progress forward and provide patients with early access to new treatments.
It’s paramount that the patient segments and investigators represented in the clinical trials be as diverse and inclusive as possible.
It Starts with Inclusion
Inclusion is the precursor to building trust between patients and doctors and the bridge leading to a more equitable healthcare system for all. Clinical operations and feasibility teams have begun to shift their approach to ensure trials are run by a more diverse group of researchers.
Both the public and private sectors have initiated efforts to address clinical trial diversity. The Food and Drug Administration (FDA) has issued guidance to encourage diversity from the design to execution of clinical product development. Meanwhile, the Pharmaceutical Research and Manufacturers of America (PhRMA) announced the release of industry-wide principles on clinical trial diversity.
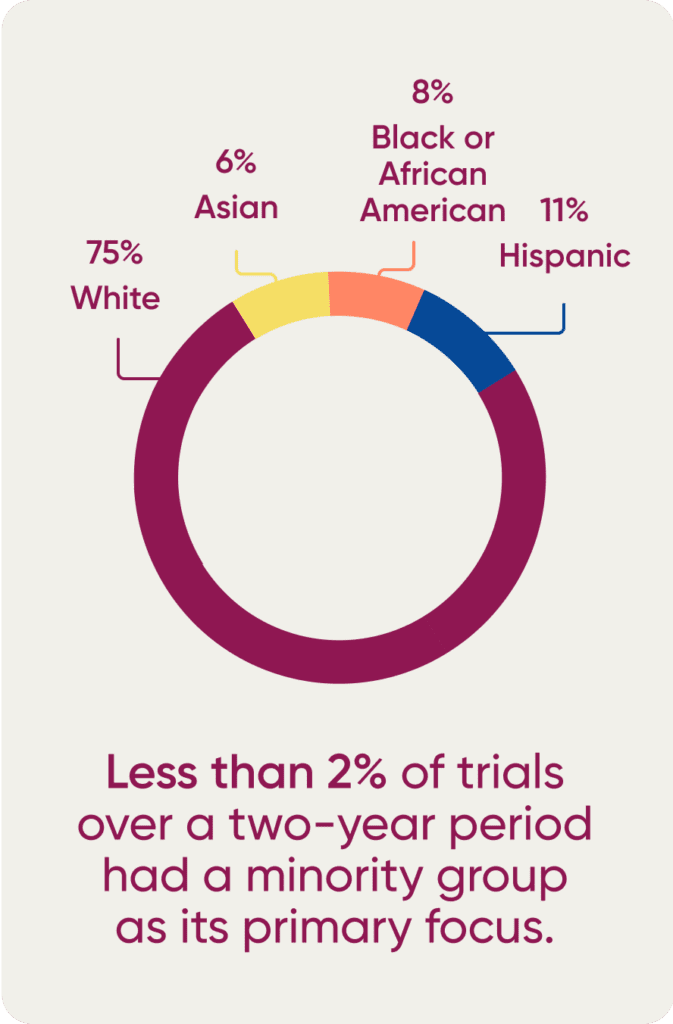
Despite these relatively recent efforts, racial and ethnic diversity in clinical trials is lacking. According to a 2021 study, less than 2% of trials over a two-year period had a minority group as its primary focus. In 2020, among 32,000 clinical trial participants, the FDA found that 75% identified as White, and only 6%, 8%, and 11% identified as Asian, Black, or Hispanic, respectively. Even the federal agency’s own efforts to improve clinical trial diversity for Black patients resulted in failure, with a five-year action plan still leading to Black individuals being underrepresented in 85% of all trials and disease categories.
Prioritizing Vulnerable Populations by Design
The first step involves addressing the overall scientific development of drugs.
Biopharmaceutical companies must test drugs in different patient populations and communities to ensure that the people receiving the approved drugs are appropriately represented during all stages of clinical development.
Differences in people can often lead to different responses to the same medication. Age, genetics, gender, weight, ethnic origin, and geographic location may play a role in treatment safety and efficacy.
Streamlining Clinical Research, Drug Development
The second piece in addressing clinical trial diversity centers on the clinical trials themselves. Suppose a new drug comes to market in the US and other regions globally. Numerous regulatory agencies require the drug to be proven safe for the patient populations receiving the drug in their specific niche.
There is a notably high benefit of initially conducting a clinical trial in a diverse patient population: Companies are no longer responsible for initiating additional trials to prove that the drug is also safe and effective in other regions.
Building Trust in Underserved Communities
The third angle is a matter of perception, namely a lingering negative perception of the biopharmaceutical industry as a whole.
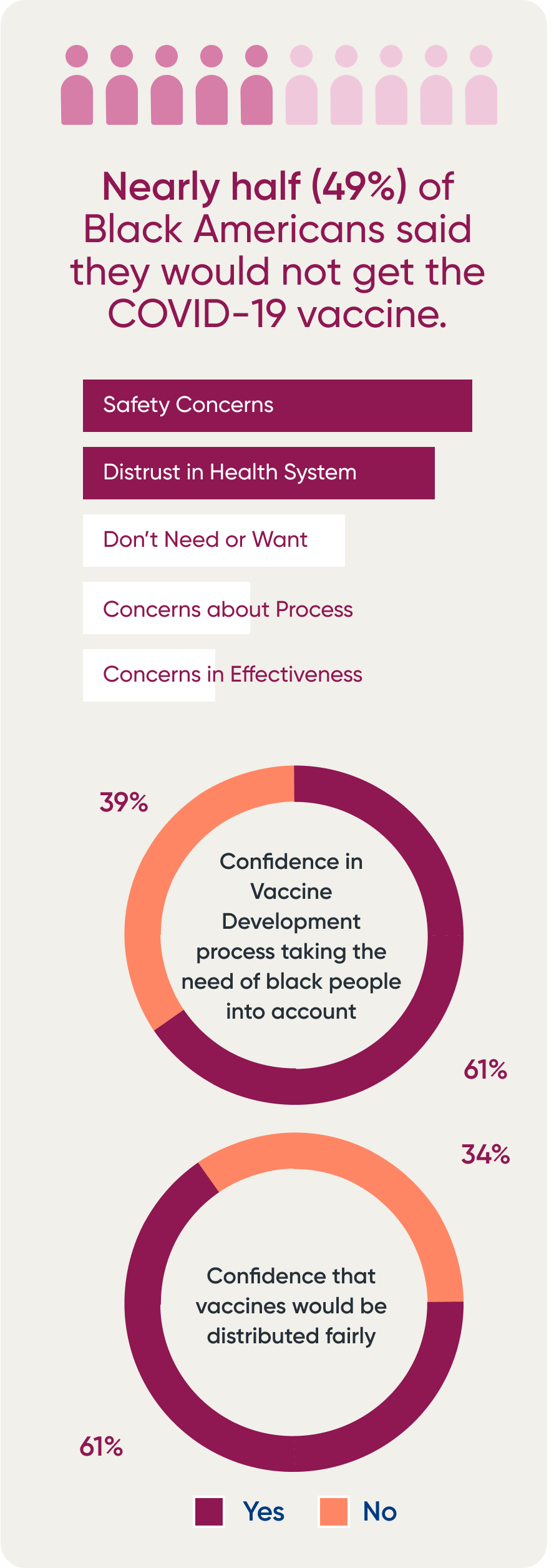
Nearly half (49%) of Black Americans said they would not get a COVID-19 vaccine, according to a 2020 Kaiser Family Foundation (KFF) report. Patients cited safety concerns (39%) and distrust (35%) as the top reasons. But a majority of Black adults (61%) had little to no confidence that the vaccine development process took the needs of Black people into account, with twothirds (66%) not confident that vaccines would be distributed fairly.
Additionally, companies that focus on good general practices and ensure that minority groups are included in trials are more likely to reposition themselves as partners in care rather than impediments or antagonists, which can help with the increasing focus on access to and equity in care.
























































































 HCP Universe
HCP Universe Trial Landscape
Trial Landscape